Dive into the intricate world of the bone marrow stroma, a complex and dynamic microenvironment that orchestrates the production of blood cells. “A Cellular Taxonomy of the Bone Marrow Stroma” delves into the diverse cellular inhabitants of this vital tissue, exploring their roles in hematopoiesis and beyond. From the enigmatic mesenchymal stromal cells (MSCs) to the vital endothelial cells lining the blood vessels, each cell type contributes to the delicate balance of this intricate system.
Understanding the cellular tapestry of the bone marrow stroma is crucial for unraveling the mysteries of blood cell development and for advancing regenerative medicine therapies.
The bone marrow stroma is more than just a passive support structure; it actively shapes and regulates the hematopoietic process. This fascinating ecosystem comprises a diverse cast of cells, each with its own unique function. Imagine a bustling city where different neighborhoods specialize in specific tasks. The bone marrow stroma is akin to this city, with specialized niches where hematopoietic stem cells (HSCs) reside, proliferate, and differentiate into various blood cell lineages.
The interplay between these cells and their surrounding microenvironment is a symphony of signals and interactions, ensuring the continuous production of healthy blood cells.
Introduction to Bone Marrow Stroma: A Cellular Taxonomy Of The Bone Marrow Stroma
:max_bytes(150000):strip_icc()/hematopoiesis-59233e485f9b58f4c05d44da.jpg)
The bone marrow stroma is a complex and dynamic microenvironment within the bone marrow, playing a crucial role in supporting hematopoiesis, the process of blood cell formation. It provides structural support, regulates hematopoietic stem cell (HSC) function, and contributes to the overall health of the bone marrow.
Historical Context of Understanding Bone Marrow Stromal Cells, A cellular taxonomy of the bone marrow stroma
The understanding of bone marrow stromal cells has evolved over time, with significant advancements in research techniques and technologies. Early observations focused on the structural components of the bone marrow, recognizing the presence of a supportive framework. As research progressed, the functional significance of the stroma in hematopoiesis became apparent. The development of cell culture techniques allowed for the isolation and characterization of specific stromal cell types, leading to a deeper understanding of their roles in regulating HSC function and blood cell development.
Cellular Components of the Bone Marrow Stroma
The bone marrow stroma is composed of a diverse population of cells, each contributing to the overall function of the microenvironment.
- Endothelial cells form the lining of blood vessels within the bone marrow, providing a conduit for nutrient and oxygen delivery to hematopoietic cells and facilitating the release of mature blood cells into circulation.
- Fibroblasts are the most abundant stromal cell type, producing extracellular matrix components such as collagen and fibronectin, which provide structural support and regulate cell adhesion and migration.
- Adipocytes are fat cells that store energy and contribute to the regulation of bone marrow niche size and function.
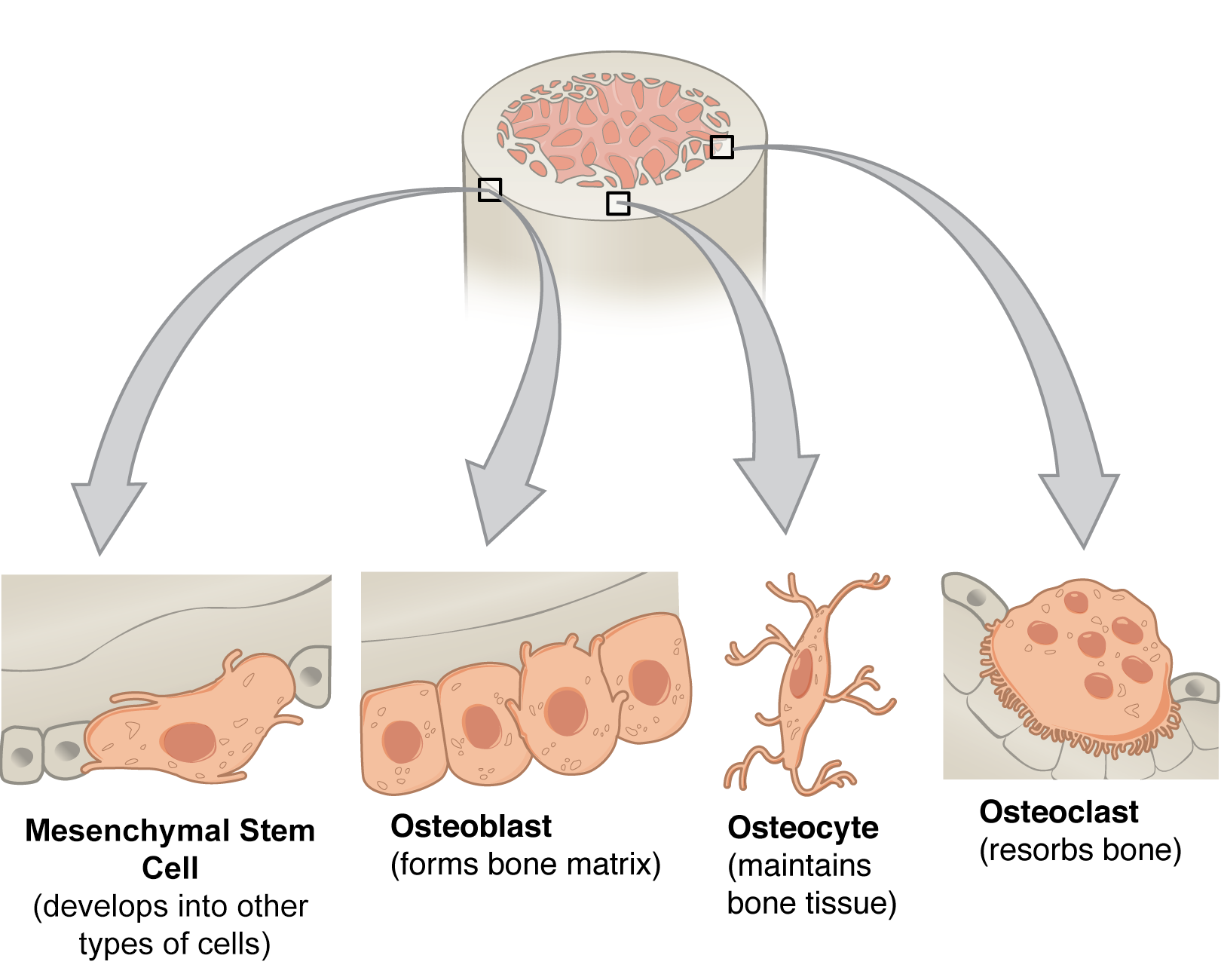
- Osteoblasts are responsible for bone formation, contributing to the structural integrity of the bone marrow cavity.
- Osteoclasts are responsible for bone resorption, playing a role in bone remodeling and the release of calcium into the bloodstream.
- Reticular cells are specialized fibroblasts that form a network of fibers providing structural support and a framework for hematopoietic cells.
- Pericytes are cells that wrap around blood vessels and contribute to vascular stability and regulation of blood flow.
- Niche-associated macrophages play a role in immune surveillance, regulation of hematopoiesis, and clearance of apoptotic cells.
Cellular Taxonomy of the Bone Marrow Stroma
The bone marrow stroma is a complex and dynamic network of cells that provides structural support and regulates the development and function of hematopoietic stem cells (HSCs). A diverse population of stromal cells, each with distinct roles, contribute to the maintenance of the hematopoietic microenvironment.
Classification of Bone Marrow Stromal Cells
The bone marrow stroma is a diverse ecosystem, with a variety of cell types, each playing a critical role in maintaining the hematopoietic microenvironment. These cells can be broadly categorized based on their lineage, function, and markers.
- Mesenchymal Stromal Cells (MSCs): MSCs are multipotent stromal cells with the capacity to differentiate into various cell types, including osteoblasts, chondrocytes, adipocytes, and myoblasts. They play a crucial role in providing structural support and regulating hematopoiesis. MSCs express a unique set of cell surface markers, including CD105, CD73, and CD90, which are used for their identification and isolation.
- Endothelial Cells: Endothelial cells line the blood vessels within the bone marrow, forming a critical interface between the blood and the hematopoietic microenvironment. They regulate the passage of nutrients, oxygen, and immune cells into and out of the bone marrow. Endothelial cells express markers such as CD31, CD144, and von Willebrand factor.
- Pericytes: Pericytes are mural cells that wrap around blood vessels and contribute to vascular stability and regulation. They play a role in the regulation of blood flow and the maintenance of the hematopoietic microenvironment. Pericytes express markers like NG2, PDGFRβ, and α-smooth muscle actin.
- Fibroblasts: Fibroblasts are responsible for producing the extracellular matrix (ECM) that provides structural support to the bone marrow. They secrete collagen, elastin, and other ECM components, creating a suitable environment for hematopoiesis. Fibroblasts express markers such as vimentin, fibronectin, and collagen type I.
- Reticular Cells: Reticular cells form a network of supporting fibers that provide structural support and a framework for hematopoiesis. They express markers like CD45, CD14, and CD11b.
- Macrophages: Macrophages are phagocytic cells that play a role in the removal of cellular debris, pathogens, and apoptotic cells from the bone marrow. They also contribute to the regulation of hematopoiesis by presenting antigens to immune cells. Macrophages express markers such as CD14, CD68, and F4/80.
Roles of Stromal Cells in Hematopoiesis
Each stromal cell type contributes to the maintenance of the hematopoietic microenvironment, regulating HSC proliferation, differentiation, and survival.
- MSCs: MSCs secrete a variety of growth factors and cytokines that support HSC proliferation and differentiation. They also provide a niche for HSCs, promoting their quiescence and self-renewal.
- Endothelial Cells: Endothelial cells regulate the passage of nutrients, oxygen, and immune cells into and out of the bone marrow, ensuring the proper functioning of hematopoiesis. They also participate in the formation of new blood vessels (angiogenesis), a crucial process for hematopoietic cell development.
- Pericytes: Pericytes contribute to the stability of blood vessels and regulate blood flow, ensuring the delivery of oxygen and nutrients to HSCs. They also participate in the regulation of HSC proliferation and differentiation.
- Fibroblasts: Fibroblasts produce the ECM that provides structural support and a framework for hematopoiesis. They also secrete growth factors and cytokines that influence HSC development.
- Reticular Cells: Reticular cells form a network of supporting fibers that provide structural support and a framework for hematopoiesis. They also participate in the regulation of HSC proliferation and differentiation.
- Macrophages: Macrophages remove cellular debris and pathogens, maintaining a healthy microenvironment for HSCs. They also present antigens to immune cells, contributing to the immune surveillance of the bone marrow.
Comparison of Stromal Cell Properties
The various stromal cell types exhibit distinct properties that contribute to their unique roles in the bone marrow.
| Cell Type | Lineage | Function | Markers |
|---|---|---|---|
| MSCs | Mesenchymal | Multipotent, support hematopoiesis | CD105, CD73, CD90 |
| Endothelial Cells | Endothelial | Regulate blood flow, support hematopoiesis | CD31, CD144, von Willebrand factor |
| Pericytes | Mural | Stabilize blood vessels, regulate hematopoiesis | NG2, PDGFRβ, α-smooth muscle actin |
| Fibroblasts | Fibroblast | Produce ECM, support hematopoiesis | Vimentin, fibronectin, collagen type I |
| Reticular Cells | Reticular | Provide structural support, support hematopoiesis | CD45, CD14, CD11b |
| Macrophages | Hematopoietic | Phagocytosis, immune surveillance | CD14, CD68, F4/80 |
Mesenchymal Stromal Cells (MSCs)
Mesenchymal stromal cells (MSCs) are multipotent stromal cells found in the bone marrow. They are characterized by their ability to self-renew and differentiate into a variety of cell types, including osteoblasts, chondrocytes, adipocytes, and myoblasts. MSCs play a crucial role in maintaining the integrity of the bone marrow microenvironment and supporting hematopoiesis.
Characteristics and Functions of MSCs in Bone Marrow
MSCs exhibit a unique set of characteristics that distinguish them from other cell types. These characteristics include:
- Multipotency: MSCs have the ability to differentiate into multiple cell lineages, including osteoblasts, chondrocytes, adipocytes, and myoblasts. This multipotency allows them to contribute to tissue repair and regeneration.
- Self-renewal: MSCs can divide and replicate themselves, maintaining a pool of undifferentiated cells for future differentiation.
- Secretion of growth factors and cytokines: MSCs produce a wide range of growth factors and cytokines that influence the behavior of other cells, including hematopoietic stem cells (HSCs).
- Immunomodulatory properties: MSCs can suppress immune responses, making them attractive candidates for cell therapy in autoimmune diseases.
In addition to their multipotency, MSCs perform various functions in the bone marrow, including:
- Support of hematopoiesis: MSCs provide a supportive microenvironment for HSCs, promoting their proliferation, differentiation, and survival. They secrete growth factors and cytokines that regulate HSC activity, ensuring the continuous production of blood cells.
- Maintenance of bone marrow structure: MSCs contribute to the structural integrity of the bone marrow by differentiating into osteoblasts, which form bone tissue.
- Tissue repair and regeneration: MSCs have the potential to differentiate into various cell types, enabling them to participate in tissue repair and regeneration after injury or disease.
Role of MSCs in Hematopoietic Stem Cell (HSC) Niche Regulation
The bone marrow microenvironment, also known as the HSC niche, provides a supportive environment for HSCs, regulating their self-renewal, differentiation, and quiescence. MSCs are integral components of the HSC niche, playing a crucial role in regulating HSC behavior.
- Direct cell-cell interactions: MSCs interact directly with HSCs through cell-cell adhesion molecules, providing physical support and signaling cues.
- Secretion of niche factors: MSCs secrete a variety of growth factors and cytokines, including stem cell factor (SCF), thrombopoietin (TPO), and interleukin-3 (IL-3), that regulate HSC proliferation, differentiation, and survival.
- Extracellular matrix production: MSCs contribute to the formation of the extracellular matrix (ECM), which provides structural support and signaling cues for HSCs.
MSCs, through their interactions with HSCs and the ECM, maintain the HSC pool, ensuring the continuous production of blood cells throughout life.
Potential of MSCs in Regenerative Medicine and Cell Therapy
The unique characteristics of MSCs, including their multipotency, self-renewal, and immunomodulatory properties, make them promising candidates for regenerative medicine and cell therapy. MSCs are being investigated for their potential to treat a wide range of diseases, including:
- Bone and cartilage regeneration: MSCs can differentiate into osteoblasts and chondrocytes, making them potential therapeutic agents for bone and cartilage defects.
- Heart disease: MSCs have shown promise in improving cardiac function after myocardial infarction by promoting angiogenesis and reducing inflammation.
- Neurological disorders: MSCs have been investigated for their potential to repair damaged neural tissue and improve neurological function in conditions such as stroke and spinal cord injury.
- Autoimmune diseases: MSCs have immunomodulatory properties, making them potential therapeutic agents for autoimmune diseases such as rheumatoid arthritis and multiple sclerosis.
Clinical trials are underway to evaluate the safety and efficacy of MSC-based therapies for various diseases. While significant progress has been made, further research is needed to optimize MSC-based therapies and ensure their long-term safety and efficacy.
Endothelial Cells and Vasculature
The bone marrow is a highly vascularized tissue, with a complex network of blood vessels that play a crucial role in supporting hematopoiesis and stromal cell function. This intricate vasculature provides a unique microenvironment that facilitates the delivery of nutrients and oxygen, the removal of waste products, and the regulation of hematopoietic stem cell (HSC) fate.
Structure and Function of the Bone Marrow Vasculature
The bone marrow vasculature is composed of a hierarchical system of blood vessels, including arterioles, capillaries, venules, and sinusoids. The sinusoids, which are specialized capillaries, are the primary site of hematopoietic cell production and release. They are characterized by their large diameter, fenestrated endothelium, and discontinuous basement membrane, allowing for the easy passage of blood cells.
- Arterioles: These small arteries deliver oxygenated blood to the bone marrow. They branch into capillaries, which further subdivide into sinusoids.
- Capillaries: These tiny blood vessels facilitate the exchange of nutrients, oxygen, and waste products between the blood and the surrounding bone marrow tissue.
- Venules: These small veins collect deoxygenated blood from the sinusoids and transport it out of the bone marrow.
- Sinusoids: These specialized capillaries are the primary site of hematopoietic cell production and release. They are characterized by their large diameter, fenestrated endothelium, and discontinuous basement membrane, allowing for the easy passage of blood cells.
Role of Endothelial Cells in Hematopoiesis and Stromal Cell Interactions
Endothelial cells, which line the blood vessels, play a critical role in hematopoiesis and stromal cell interactions. They contribute to the regulation of HSC fate, provide a niche for hematopoietic cell development, and participate in the formation of the bone marrow microenvironment.
- HSC Niche: Endothelial cells create a specialized microenvironment, known as the HSC niche, which provides signals and support for HSC self-renewal and differentiation. This niche is characterized by specific cell-cell interactions, soluble factors, and extracellular matrix components that regulate HSC fate.
- Hematopoietic Cell Development: Endothelial cells secrete a variety of growth factors and cytokines that promote the proliferation, differentiation, and survival of hematopoietic cells. These factors include vascular endothelial growth factor (VEGF), thrombopoietin (TPO), and stem cell factor (SCF), which are essential for hematopoiesis.
- Stromal Cell Interactions: Endothelial cells interact with other stromal cells, such as mesenchymal stem cells (MSCs), to maintain the integrity and functionality of the bone marrow microenvironment. These interactions involve the exchange of signaling molecules and the formation of specialized cell-cell junctions.
Contribution of Blood Vessels to the Regulation of HSC Fate
Blood vessels play a crucial role in the regulation of HSC fate by providing a dynamic and responsive environment that influences HSC self-renewal, differentiation, and mobilization. This regulation is achieved through a complex interplay of factors, including mechanical forces, soluble factors, and cell-cell interactions.
- Mechanical Forces: Blood flow and shear stress exerted by blood vessels can influence HSC fate. These forces can stimulate HSC proliferation and differentiation, and contribute to the maintenance of HSC quiescence.
- Soluble Factors: Blood vessels secrete a variety of soluble factors, such as VEGF, TPO, and SCF, that regulate HSC fate. These factors can influence HSC self-renewal, differentiation, and mobilization.
- Cell-Cell Interactions: Endothelial cells interact with HSCs and other stromal cells to regulate HSC fate. These interactions involve the exchange of signaling molecules and the formation of specialized cell-cell junctions.
Pericytes and Their Role
Pericytes, also known as Rouget cells, are perivascular cells that wrap around the endothelial cells of capillaries and venules, forming a network that supports and regulates the microvasculature. They are a critical component of the bone marrow stroma, contributing to the structural integrity of blood vessels and influencing hematopoiesis.
Location and Structure
Pericytes are located in close proximity to the endothelial cells lining the blood vessels, residing within the basement membrane that surrounds the vessel. They exhibit a characteristic elongated, spindle-shaped morphology with numerous cytoplasmic processes that extend along the vessel wall. These processes, rich in actin filaments, enable pericytes to contract and regulate blood flow.
Functions of Pericytes
Pericytes play crucial roles in maintaining blood vessel stability and regulating hematopoiesis.
Blood Vessel Stability
- Contractility: Pericytes possess contractile properties, allowing them to regulate blood flow through vasoconstriction and vasodilation. This dynamic regulation is essential for maintaining tissue perfusion and oxygenation.
- Structural Support: Pericytes provide structural support to the blood vessel wall, contributing to its integrity and stability. Their close association with the basement membrane and endothelial cells helps to maintain the vessel’s permeability and prevent leakage.
- Angiogenesis: Pericytes actively participate in angiogenesis, the formation of new blood vessels. They promote vessel sprouting and maturation, ensuring adequate blood supply to growing tissues.
Hematopoiesis
- Niche Regulation: Pericytes create a specialized microenvironment, or niche, that supports hematopoietic stem cell (HSC) survival, proliferation, and differentiation. They secrete factors that regulate HSC behavior, including chemokines, growth factors, and extracellular matrix components.
- HSC Maintenance: Pericytes contribute to the maintenance of HSC quiescence, a state of dormancy that preserves HSC function and longevity. This is essential for ensuring long-term hematopoietic capacity.
- Hematopoietic Cell Differentiation: Pericytes influence the differentiation of HSCs into various blood cell lineages, including erythrocytes, leukocytes, and platelets. They secrete factors that promote specific lineage commitment and development.
Interaction with Other Stromal Cells
Pericytes engage in complex interactions with other stromal cells, including mesenchymal stromal cells (MSCs), endothelial cells, and macrophages.
MSCs
Pericytes and MSCs exhibit reciprocal interactions, influencing each other’s function and fate. Pericytes can stimulate MSC proliferation and differentiation, contributing to the generation of new stromal cells. Conversely, MSCs can secrete factors that promote pericyte survival and angiogenesis.
Endothelial Cells
Pericytes and endothelial cells form a close functional unit, working together to maintain blood vessel integrity and regulate blood flow. Pericytes provide structural support to endothelial cells, while endothelial cells secrete factors that promote pericyte survival and differentiation.
Macrophages
Pericytes interact with macrophages, immune cells that reside within the bone marrow stroma. Pericytes can recruit macrophages to sites of injury or inflammation, where they contribute to tissue repair and immune regulation.
Immune Cells in the Bone Marrow Stroma
The bone marrow stroma is not only a supportive framework for hematopoiesis but also a dynamic environment teeming with immune cells. These cells play crucial roles in regulating hematopoiesis, orchestrating immune responses, and maintaining tissue homeostasis.
Types of Immune Cells in the Bone Marrow Stroma
Immune cells in the bone marrow stroma are diverse and contribute to the complex interplay of hematopoiesis and immunity.
- T lymphocytes (T cells): These cells are essential for adaptive immunity, recognizing and eliminating specific pathogens. They differentiate into various subtypes, including helper T cells (Th), cytotoxic T cells (Tc), and regulatory T cells (Treg).
- B lymphocytes (B cells): These cells are responsible for humoral immunity, producing antibodies to neutralize pathogens. They mature into plasma cells, which secrete antibodies.
- Natural killer (NK) cells: These cells are part of the innate immune system, targeting and eliminating infected or cancerous cells.
- Macrophages: These phagocytic cells engulf and digest cellular debris, pathogens, and foreign substances. They also present antigens to T cells, contributing to adaptive immunity.
- Dendritic cells (DCs): These antigen-presenting cells capture antigens and migrate to lymph nodes, where they present them to T cells, initiating adaptive immune responses.
- Neutrophils: These are the most abundant type of white blood cells, playing a crucial role in innate immunity by phagocytosing bacteria and fungi.
Role of Immune Cells in Hematopoiesis and Immune Surveillance
Immune cells in the bone marrow stroma contribute significantly to both hematopoiesis and immune surveillance.
- Regulation of Hematopoiesis: Immune cells, particularly T cells and macrophages, influence hematopoiesis by releasing cytokines that regulate the differentiation and proliferation of hematopoietic stem cells (HSCs).
- Immune Surveillance: The bone marrow is a site of constant immune surveillance, with immune cells patrolling the stroma to detect and eliminate pathogens, cancerous cells, or abnormal hematopoietic cells.
Interactions Between Immune Cells and Other Stromal Components
Immune cells within the bone marrow stroma engage in intricate interactions with other stromal components.
- MSCs: Immune cells, especially T cells and macrophages, interact with MSCs. MSCs can modulate immune responses by secreting cytokines and chemokines, influencing the differentiation and activation of immune cells.
- Endothelial Cells: Immune cells interact with endothelial cells, contributing to the regulation of blood vessel formation and permeability.
- Extracellular Matrix (ECM): Immune cells interact with the ECM, influencing their migration, adhesion, and activation.
Extracellular Matrix (ECM) and Its Importance
The bone marrow stroma is not just a collection of cells; it also contains a complex and dynamic network of extracellular matrix (ECM) components. This intricate web of molecules plays a crucial role in providing structural support, regulating cell behavior, and orchestrating the essential processes of hematopoiesis and stromal cell function.
Composition and Structure of the Bone Marrow ECM
The bone marrow ECM is a diverse mixture of proteins, carbohydrates, and other molecules, forming a scaffold that provides structural support and regulates cell interactions. The key components include:
- Collagen: This fibrous protein is the most abundant component of the ECM, providing tensile strength and forming a framework for the stroma. Type I collagen is the predominant type, but other collagens, such as type III and type IV, are also present.
- Fibronectin: This glycoprotein acts as a bridge between cells and the ECM, facilitating cell adhesion and migration. It also binds to other ECM components, contributing to the overall structural integrity of the stroma.
- Laminin: This protein forms a sheet-like structure, providing structural support and influencing cell differentiation. It also plays a role in the formation of basement membranes, which surround blood vessels and other structures within the bone marrow.
- Proteoglycans: These molecules consist of a core protein attached to long chains of glycosaminoglycans (GAGs). Proteoglycans, like hyaluronic acid and chondroitin sulfate, contribute to the structural integrity of the ECM and regulate water content, influencing the viscosity and diffusion properties of the bone marrow microenvironment.
- Other ECM Components: The bone marrow ECM also contains other important molecules, such as elastin, which provides elasticity, and growth factors, which influence cell proliferation and differentiation.
Role of the ECM in Providing Structural Support and Signaling Cues
The ECM provides a physical scaffold that supports the bone marrow microenvironment, ensuring the proper organization and function of its cellular components. The ECM also acts as a reservoir for growth factors and other signaling molecules, influencing cell behavior and regulating various processes within the bone marrow.
Contribution of the ECM to the Regulation of Hematopoiesis and Stromal Cell Function
The ECM plays a critical role in hematopoiesis, the process of blood cell formation. The ECM provides a niche for hematopoietic stem cells (HSCs), regulating their proliferation, differentiation, and self-renewal. The ECM also influences the development and function of stromal cells, including MSCs, which contribute to the hematopoietic microenvironment.
- Regulation of HSC Niche: The ECM components, such as collagen and fibronectin, provide a scaffold for the HSC niche, a specialized microenvironment that supports HSC survival, self-renewal, and differentiation.
- Influence on MSC Function: The ECM regulates MSC behavior, influencing their proliferation, differentiation, and production of growth factors and cytokines that support hematopoiesis.
- Modulation of Hematopoietic Cell Development: The ECM interacts with hematopoietic cells, influencing their differentiation and maturation into various blood cell types. For instance, the ECM can provide specific cues that promote the development of specific lineages, such as erythrocytes or lymphocytes.
Bone Marrow Stroma and Disease
The bone marrow stroma, a complex and dynamic network of cells and extracellular matrix, plays a critical role in maintaining hematopoiesis and overall bone marrow function. However, disruptions in stromal integrity can lead to various diseases, including hematological malignancies and bone marrow failure syndromes. Understanding the intricate interplay between stromal cells and disease pathogenesis is crucial for developing effective therapeutic strategies.
Hematological Malignancies and Stromal Cells
Stromal cells are intimately involved in the development and progression of hematological malignancies, such as leukemia, lymphoma, and myeloma. Malignant cells often exploit the supportive microenvironment provided by the stroma for their survival, proliferation, and drug resistance.
- Niche Formation: Stromal cells create specialized niches that provide essential growth factors, cytokines, and adhesion molecules that promote the survival and proliferation of malignant cells. These niches can act as sanctuaries for cancer cells, shielding them from the effects of chemotherapy and other therapies.
- Immune Evasion: Stromal cells can suppress the immune response against malignant cells by producing immunosuppressive factors or by directly interacting with immune cells. This immune evasion mechanism contributes to the persistence and spread of cancer.
- Drug Resistance: Stromal cells can induce drug resistance in malignant cells by altering their metabolism, reducing drug uptake, or promoting DNA repair mechanisms. This resistance further complicates treatment strategies.
Stromal Dysfunction in Bone Marrow Failure Syndromes
Bone marrow failure syndromes, such as aplastic anemia and myelodysplastic syndromes, are characterized by a deficiency in hematopoietic stem cells and impaired blood cell production. Stromal dysfunction plays a significant role in these disorders, contributing to the depletion of hematopoietic stem cells and the overall failure of bone marrow function.
- Microenvironment Alterations: Stromal cells in bone marrow failure syndromes often exhibit abnormal morphology, reduced production of growth factors, and altered expression of adhesion molecules. These changes disrupt the normal hematopoietic microenvironment, leading to impaired stem cell function and differentiation.
- Immune Dysregulation: The immune system can also contribute to stromal dysfunction in bone marrow failure syndromes. Autoreactive T cells can target stromal cells, leading to their damage and dysfunction. This immune-mediated destruction of stromal cells further exacerbates the hematopoietic defect.
- Genetic Predisposition: Some bone marrow failure syndromes are associated with specific genetic mutations that affect stromal cell function. These mutations can disrupt signaling pathways involved in stromal cell development, survival, and function, ultimately contributing to the disease phenotype.
Targeting Stromal Cells for Therapeutic Interventions
The critical role of stromal cells in hematological malignancies and bone marrow failure syndromes has led to the development of therapeutic strategies that target these cells. These strategies aim to modulate stromal cell function and restore normal hematopoiesis.
- Stromal Cell Transplantation: Transplantation of healthy stromal cells can provide a supportive microenvironment for hematopoietic stem cells, promoting their engraftment and differentiation. This approach is particularly promising for patients with bone marrow failure syndromes.
- Stromal Cell Modulation: Various pharmacological agents are being investigated to modulate stromal cell function, either by inhibiting their support of malignant cells or by promoting their regenerative properties. These agents can target specific signaling pathways or receptors involved in stromal cell activity.
- Immunotherapy: Immunotherapeutic strategies aim to restore immune function and target malignant cells within the stromal microenvironment. These strategies can involve the use of antibodies, CAR T cells, or other immune modulators to eliminate cancer cells or re-educate the immune system to recognize and attack them.
Future Directions in Bone Marrow Stroma Research
:max_bytes(150000):strip_icc()/what-is-bone-marrow-5083764-v1-15c1057db07a44ea9981594d460e7e8d.jpg)
The bone marrow stroma, a complex and dynamic microenvironment, plays a crucial role in hematopoiesis and other physiological processes. Understanding its intricate structure and function is essential for advancing therapeutic strategies in hematological disorders and regenerative medicine. Emerging technologies and innovative approaches are revolutionizing our understanding of the bone marrow stroma, paving the way for groundbreaking discoveries and clinical applications.
The Potential of Single-Cell Analysis and Bioinformatics
Single-cell analysis, a powerful tool for studying cellular heterogeneity, has emerged as a transformative approach in bone marrow stroma research. It allows researchers to analyze individual cells within the complex microenvironment, providing unprecedented insights into the diverse populations of stromal cells, their interactions, and their contributions to hematopoiesis.
- Single-cell RNA sequencing (scRNA-seq) enables the profiling of gene expression in individual cells, revealing unique molecular signatures and identifying novel cell subtypes within the bone marrow stroma. This approach has already led to the discovery of previously unknown stromal cell populations with distinct functions and developmental origins.
- Single-cell proteomics, using techniques such as mass spectrometry, provides a snapshot of the protein landscape within individual stromal cells. This allows researchers to study protein expression, post-translational modifications, and protein-protein interactions, providing a deeper understanding of cellular function and signaling pathways.
- Single-cell spatial transcriptomics combines the power of scRNA-seq with spatial information, enabling researchers to map the location and gene expression of individual cells within the bone marrow stroma. This technology is crucial for understanding the spatial organization of stromal cells and their interactions with hematopoietic stem cells and other cell types.
Bioinformatics tools are essential for analyzing the vast amounts of data generated by single-cell technologies. Advanced algorithms and statistical methods are used to identify cell clusters, analyze gene expression patterns, and infer cellular interactions. This computational approach facilitates the identification of key regulatory pathways and biomarkers, paving the way for targeted therapies and personalized medicine.
“Single-cell analysis, coupled with bioinformatics, is revolutionizing our understanding of the bone marrow stroma. It provides unprecedented insights into the cellular diversity, functional heterogeneity, and complex interactions within this microenvironment.”
As we conclude our journey through “A Cellular Taxonomy of the Bone Marrow Stroma,” we are left with a profound appreciation for the complexity and elegance of this vital tissue. The intricate interplay between stromal cells and hematopoietic cells reveals a symphony of signaling pathways and regulatory mechanisms that govern the production of our lifeblood. Further exploration of the bone marrow stroma holds immense promise for understanding hematological diseases, developing novel therapies, and harnessing the regenerative potential of stromal cells.
Detailed FAQs
What is the difference between bone marrow stroma and hematopoietic cells?
Bone marrow stroma refers to the supporting framework of the bone marrow, composed of various cell types and extracellular matrix. Hematopoietic cells are the blood-forming cells that reside within the stroma, responsible for producing all blood cell types.
What are the potential applications of bone marrow stromal cells in regenerative medicine?
MSCs, a key component of the bone marrow stroma, hold great promise in regenerative medicine due to their ability to differentiate into various cell types and modulate immune responses. They are being investigated for treating a wide range of conditions, including bone and cartilage defects, cardiovascular diseases, and autoimmune disorders.
How does the bone marrow stroma contribute to immune surveillance?
The bone marrow stroma plays a crucial role in immune surveillance by housing and regulating various immune cells, including lymphocytes and macrophages. These cells patrol the bone marrow, detecting and eliminating pathogens or abnormal cells, ensuring the integrity of the hematopoietic system.
:max_bytes(150000):strip_icc()/hematopoiesis-59233e485f9b58f4c05d44da.jpg?w=1024&resize=1024,1024&ssl=1)





