A patterned human neural tube model using microfluidic gradients represents a significant advancement in developmental biology and neuroscience research. Traditional methods for studying neural tube development have limitations, often relying on static models that lack the dynamic and intricate features of the developing embryo. Microfluidic gradients, however, offer a powerful tool for creating patterned tissues, mimicking the complex signaling pathways and spatial cues that guide neural tube formation.
This innovative approach allows researchers to delve deeper into the intricacies of neural tube development, providing valuable insights into the underlying mechanisms that govern this crucial process.
Microfluidic gradients leverage the principles of fluid dynamics to create precise and controlled gradients of various factors, such as growth factors, chemokines, or even temperature. These gradients can be engineered to mimic the natural gradients present in the developing embryo, influencing cell fate and patterning in a highly controlled manner. By manipulating these gradients, researchers can create patterned tissues that resemble the complex organization of the neural tube, providing a powerful tool for investigating the role of specific signaling pathways in neural tube development.
Introduction: A Patterned Human Neural Tube Model Using Microfluidic Gradients
The intricate process of human neural tube development is a captivating area of research in developmental biology and neuroscience. Understanding the mechanisms that govern this complex process is crucial for unraveling the origins of neurological disorders and for developing effective therapeutic strategies. However, traditional methods for studying neural tube development often face limitations, making it challenging to gain a comprehensive understanding of this intricate process.Traditional methods for studying neural tube development, such as animal models and cell culture experiments, have provided valuable insights but are often limited by their complexity and lack of control over environmental factors.
Animal models can be expensive and time-consuming, and they may not fully recapitulate human development. Cell culture experiments, while offering greater control, often fail to capture the intricate interactions and spatial gradients that characterize neural tube formation in vivo.
Microfluidic Gradients and Patterned Neural Tube Models
Microfluidic gradients offer a promising approach to overcome these limitations. Microfluidics is a field that manipulates fluids at the microscale, enabling precise control over the composition and distribution of molecules within microfluidic devices. This technology allows for the creation of gradients of signaling molecules, such as growth factors and morphogens, that mimic the natural gradients present during neural tube development.By leveraging microfluidic gradients, researchers can create patterned neural tube models that more accurately reflect the complexity of in vivo development.
These models provide a platform to study the effects of specific signaling molecules on neural tube patterning, cell fate determination, and the formation of different neural cell types.
Microfluidic Gradient Technology
Microfluidic gradient technology is a powerful tool for creating patterned tissues. It utilizes microfluidic devices to generate precise and controlled gradients of various factors, including chemical concentrations, temperature, and even mechanical forces. These gradients play a crucial role in guiding cell behavior and tissue development, enabling the formation of complex and functional tissue structures.
Types of Microfluidic Gradients
Microfluidic gradients can be categorized based on the factor being varied.
- Concentration gradients: These gradients involve the controlled variation of the concentration of a specific chemical or biological factor along the microfluidic channel. This is achieved by introducing solutions with different concentrations at specific points within the device, allowing for a gradual change in concentration along the channel. Concentration gradients are widely used to study cell responses to varying levels of growth factors, drugs, or other stimuli.
- Temperature gradients: These gradients involve the creation of a controlled temperature difference along the microfluidic channel. This can be achieved by using integrated heating elements or by controlling the temperature of the surrounding environment. Temperature gradients are used to study the effects of temperature on cell behavior and to create spatially distinct environments for cell growth and differentiation.
- Mechanical gradients: These gradients involve the creation of a controlled variation in mechanical forces, such as shear stress or pressure, along the microfluidic channel. These forces can be generated by fluid flow or by using microfabricated structures within the channel. Mechanical gradients are used to study the effects of mechanical forces on cell behavior and to mimic the physical environments found in tissues.
Advantages of Microfluidic Gradients for Patterned Tissues
Microfluidic gradients offer several advantages for creating patterned tissues:
- Precise control: Microfluidic devices allow for precise control over the spatial and temporal gradients of various factors, enabling the generation of complex and reproducible patterns. This level of control is difficult to achieve using traditional methods.
- High throughput: Microfluidic devices can be easily replicated and integrated into high-throughput systems, allowing for the simultaneous generation of multiple tissue patterns. This is beneficial for screening different conditions and for studying the effects of various factors on tissue development.
- High resolution: Microfluidic devices can generate gradients with high spatial resolution, enabling the creation of fine-scale patterns within tissues. This allows for the generation of complex tissue structures that mimic the organization found in native tissues.
- In vitro mimicking: Microfluidic gradients can be used to create microenvironments that mimic the physical and chemical gradients found in native tissues. This allows for the study of cell behavior in more physiologically relevant conditions.
Design and Fabrication of the Microfluidic Device

The microfluidic device serves as the platform for generating the patterned neural tube model. Its design is crucial for achieving precise control over the microenvironment and ensuring successful patterning of the neural cells. This section details the design considerations, materials, and fabrication techniques employed in the construction of the microfluidic device.
Device Design
The microfluidic device consists of two main parts: a microfluidic channel and a patterned substrate. The microfluidic channel is responsible for delivering the gradient of growth factors to the neural cells, while the patterned substrate provides a spatially defined surface for cell adhesion and differentiation.
- Microfluidic Channel: The microfluidic channel is designed to generate a linear concentration gradient of growth factors along its length. The channel is typically fabricated using polydimethylsiloxane (PDMS), a biocompatible and elastomeric material known for its ease of fabrication and optical transparency. The channel dimensions are carefully chosen to ensure efficient diffusion of the growth factors and to allow for sufficient cell seeding and growth.
The channel is typically several millimeters long and a few hundred micrometers wide, with a depth of tens of micrometers.
- Patterned Substrate: The patterned substrate is designed to guide the spatial organization of the neural cells. It is fabricated using a variety of techniques, including photolithography, soft lithography, or microcontact printing. The substrate is typically made of a biocompatible material such as glass, silicon, or a polymer. The pattern consists of features that promote cell adhesion, such as microgrooves or microdots, which can be arranged in a specific geometry to influence cell morphology and differentiation.
Fabrication Techniques
The fabrication of the microfluidic device involves several steps, including:
- Master Mold Fabrication: The first step involves creating a master mold using photolithography. A photoresist is spin-coated onto a silicon wafer and patterned using a UV light exposure through a mask. The exposed photoresist is then developed, leaving behind a raised pattern that serves as the mold for the microfluidic channel.
- PDMS Casting: PDMS prepolymer is poured onto the master mold and cured at elevated temperature. Once cured, the PDMS replica is peeled off the master mold, resulting in a microfluidic channel with the desired geometry.
- Substrate Patterning: The patterned substrate is fabricated using a suitable technique, such as photolithography, soft lithography, or microcontact printing. In photolithography, a photoresist is patterned on a substrate using UV light exposure through a mask. In soft lithography, a PDMS mold is used to transfer a pattern onto the substrate. Microcontact printing involves transferring a pattern from a patterned elastomer stamp onto the substrate.
- Device Assembly: The patterned substrate is then bonded to the PDMS microfluidic channel using plasma treatment. The plasma treatment creates a hydrophilic surface on both the PDMS and the substrate, enabling them to bond together.
Design Rationale
The specific design choices for the microfluidic device are driven by the following considerations:
- Control over Gradient Formation: The microfluidic channel is designed to generate a stable and reproducible concentration gradient of growth factors. The channel dimensions and flow rate are carefully chosen to ensure efficient diffusion of the growth factors and to minimize mixing effects. The linear gradient is preferred for mimicking the natural gradients encountered by neural cells during development.
- Spatial Patterning of Neural Cells: The patterned substrate provides a spatially defined environment for cell adhesion and differentiation. The features on the substrate, such as microgrooves or microdots, can influence cell morphology and differentiation, leading to the formation of specific neural cell types and structures. For example, microgrooves can guide the alignment of neurons along their long axis, while microdots can promote the formation of neuronal clusters.
- Biocompatibility and Optical Transparency: The materials used in the microfluidic device are chosen for their biocompatibility and optical transparency. PDMS is a biocompatible material that allows for the observation of cells and their behavior under the microscope. Glass and silicon are also commonly used materials for their biocompatibility and optical properties.
- Ease of Fabrication: The fabrication techniques are chosen for their ease of implementation and scalability. Photolithography and soft lithography are well-established techniques that can be used to fabricate microfluidic devices with high precision and reproducibility. The use of PDMS as the microfluidic channel material further simplifies the fabrication process.
Cell Culture and Differentiation
The successful generation of a patterned neural tube model using microfluidic gradients requires the use of specific cell types and well-defined culture conditions to promote their differentiation into neural progenitors and ultimately, neural tube-like structures. This section delves into the cell types employed, the culture protocols, and the differentiation strategies used to achieve this complex developmental process.
Cell Types, A patterned human neural tube model using microfluidic gradients
The choice of cell type is crucial for generating a functional and representative neural tube model. Human embryonic stem cells (hESCs) and induced pluripotent stem cells (iPSCs) are commonly used due to their ability to differentiate into all cell types of the body, including neural cells.
- Human Embryonic Stem Cells (hESCs): These cells are derived from the inner cell mass of a blastocyst, the early stage of an embryo. They possess the unique capability of self-renewal and pluripotency, allowing them to differentiate into any cell type of the body. hESCs have been widely used in neural tube modeling due to their inherent developmental potential and the availability of established protocols for neural differentiation.
- Induced Pluripotent Stem Cells (iPSCs): These cells are generated by reprogramming adult somatic cells, such as skin fibroblasts, into a pluripotent state using specific transcription factors. iPSCs offer an attractive alternative to hESCs as they can be derived from patients, enabling the study of disease-specific neural development. Additionally, ethical concerns associated with hESCs are mitigated by the use of iPSCs.
Cell Culture Protocols
Maintaining the pluripotency and viability of hESCs and iPSCs requires specific culture conditions and media formulations.
- Media Composition: Culture media for hESCs and iPSCs typically contain a mixture of essential nutrients, growth factors, and inhibitors. For instance, media for hESCs often include basic fibroblast growth factor (bFGF) to promote self-renewal, while inhibitors like Y-27632 are added to prevent premature differentiation. Media for iPSCs might include additional components like TGF-β inhibitors to enhance their pluripotency.
- Growth Conditions: hESCs and iPSCs are usually cultured on feeder cells, such as mouse embryonic fibroblasts (MEFs), or on specialized substrates like Matrigel. These substrates provide a suitable environment for cell attachment and proliferation. The culture conditions are typically maintained in a humidified incubator with 5% CO 2 at 37°C.
Neural Differentiation Protocol
The differentiation of hESCs or iPSCs into neural tube-like structures requires a carefully orchestrated process that mimics the developmental events occurring during embryonic development.
- Neural Induction: The initial step involves the induction of neural progenitors from the pluripotent cells. This is achieved by exposing the cells to specific growth factors and signaling molecules. For example, a combination of bone morphogenetic protein (BMP) inhibitors and fibroblast growth factor (FGF) can effectively induce neural fate.
- Neural Tube Formation: After neural induction, the cells undergo a series of developmental events, including the formation of neural rosettes, which are structures resembling the early stages of neural tube formation. This process is influenced by factors like sonic hedgehog (Shh) signaling, which plays a critical role in neural tube patterning and development.
- Patterning and Differentiation: Further differentiation into specific neural cell types, such as neurons, astrocytes, and oligodendrocytes, can be achieved by manipulating the culture conditions. This might involve the use of additional growth factors, morphogens, or small molecules that mimic the natural cues present during neural development.
Patterned Neural Tube Formation
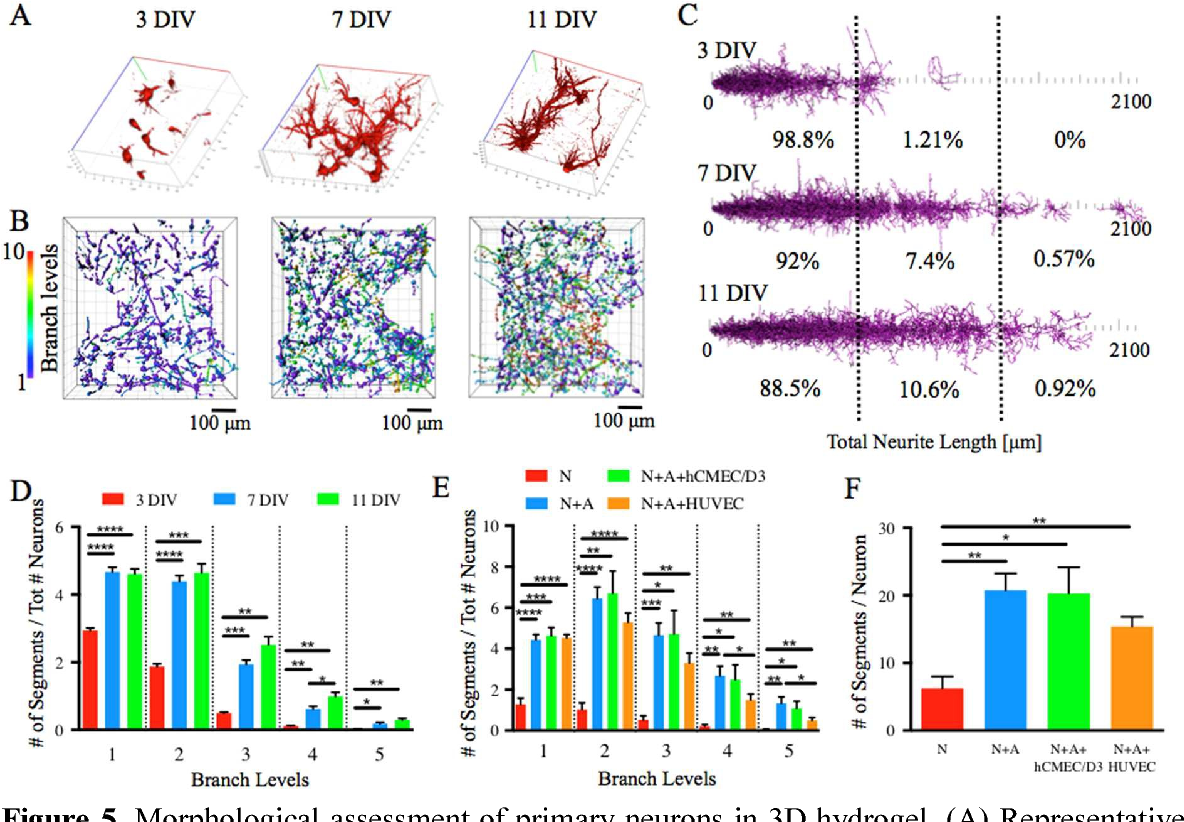
This section explores the influence of microfluidic gradients on cell fate and patterning during neural tube formation within our model system. We demonstrate the formation of distinct cell populations within the patterned neural tube model and compare it to control models lacking microfluidic gradients.
Evidence of Distinct Cell Populations
The microfluidic gradients created in our device effectively direct the differentiation of neural progenitor cells (NPCs) into distinct cell populations within the patterned neural tube model. These gradients, established by varying concentrations of morphogens, play a crucial role in defining cell fate and spatial organization.
- Dorsal-Ventral Patterning: The gradient of Shh, a morphogen essential for ventral neural patterning, promotes the differentiation of NPCs into ventral cell types, including motor neurons and interneurons. Conversely, the gradient of BMP, a morphogen that influences dorsal patterning, promotes the differentiation of NPCs into dorsal cell types, such as sensory neurons and glial cells.
- Formation of Distinct Cell Layers: The patterned neural tube model exhibits the formation of distinct cell layers, reflecting the complex organization of the developing neural tube. The ventral region, exposed to higher concentrations of Shh, displays a higher density of motor neurons, while the dorsal region, exposed to higher concentrations of BMP, exhibits a higher density of sensory neurons.
Comparison with Control Models
To assess the effectiveness of microfluidic gradients in patterning the neural tube, we compared our patterned model to control models lacking microfluidic gradients.
- Control Models: Control models, cultured in uniform media without gradients, displayed a more homogenous cell population, lacking the distinct dorsal-ventral patterning observed in the patterned model.
- Improved Patterning: The patterned neural tube model, exposed to microfluidic gradients, exhibited a significantly enhanced degree of cell differentiation and spatial organization, demonstrating the critical role of gradients in neural tube development.
Functional Characterization
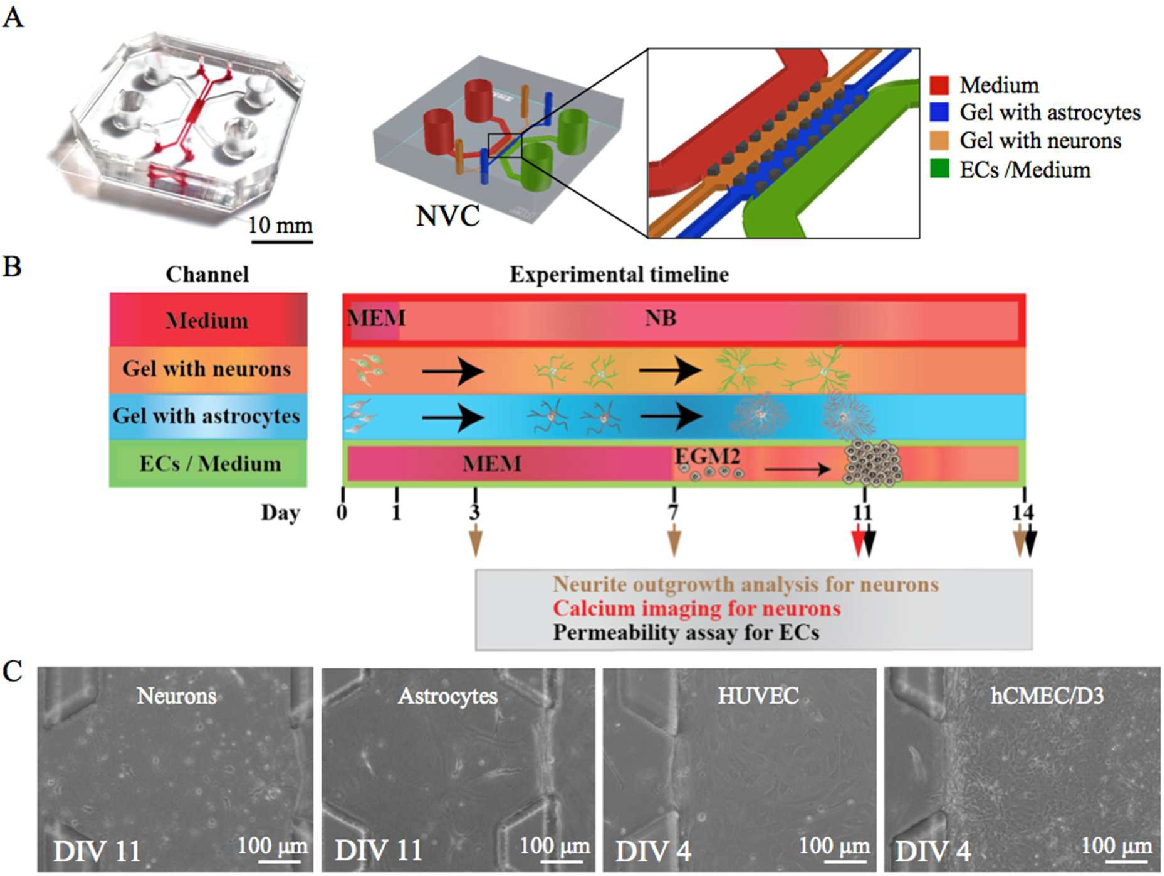
The functional characterization of the patterned neural tube model is crucial to assess its ability to mimic the complex processes occurring during neural development. This involves evaluating the model’s capacity to generate functional neurons, establish neuronal networks, and respond to stimuli.
Neural Marker Expression and Neuronal Network Formation
To assess the functional properties of the patterned neural tube model, a comprehensive analysis of neural marker expression and neuronal network formation is conducted. This analysis aims to determine the extent to which the model recapitulates the key features of neural development, providing insights into its potential for studying neural tube defects and other developmental disorders.
- Immunofluorescence Staining: Immunofluorescence staining is employed to detect the expression of specific neural markers, such as microtubule-associated protein 2 (MAP2), a marker for mature neurons, and glial fibrillary acidic protein (GFAP), a marker for astrocytes. The presence and distribution of these markers provide evidence for the differentiation of neural progenitor cells into functional neuronal subtypes.
- Electrophysiological Recordings: Electrophysiological recordings, such as patch-clamp recordings, are performed to assess the electrical activity of neurons within the patterned neural tube model. This technique allows for the measurement of action potentials and synaptic transmission, providing insights into the functional connectivity of the neuronal network.
- Calcium Imaging: Calcium imaging is a powerful technique used to visualize neuronal activity in real-time. By monitoring the changes in intracellular calcium levels, researchers can track the propagation of action potentials and synaptic communication within the neuronal network. This technique provides valuable information about the dynamic interactions between neurons and the functional integrity of the network.
Evaluation of Model Potential for Studying Neural Tube Defects
The patterned neural tube model provides a valuable platform for studying neural tube defects (NTDs), a group of birth defects that occur when the neural tube fails to close completely during embryonic development.
- Exposure to Teratogens: The model can be used to investigate the effects of teratogens, environmental agents that can cause birth defects, on neural tube development. By exposing the model to specific teratogens, researchers can observe the resulting alterations in neural tube patterning and formation, providing insights into the mechanisms underlying NTDs.
- Genetic Mutations: The model can be utilized to study the effects of genetic mutations associated with NTDs. By introducing specific genetic mutations into the model, researchers can investigate how these mutations disrupt neural tube development and lead to NTDs.
- Drug Screening: The patterned neural tube model offers a platform for drug screening to identify potential therapeutic agents for NTDs. By testing different compounds on the model, researchers can assess their ability to prevent or mitigate NTDs, leading to the development of new treatment strategies.
The development of a patterned human neural tube model using microfluidic gradients marks a significant leap forward in our understanding of neural tube development. This innovative approach offers unprecedented control over the microenvironment, allowing researchers to study the intricate interplay of signaling pathways and cell fate decisions that govern this crucial process. This technology holds immense potential for advancing our understanding of neural tube defects, paving the way for novel therapeutic strategies.
By providing a platform for studying the complexities of neural tube development in a highly controlled and dynamic manner, this patterned model promises to revolutionize our understanding of this fundamental biological process.
Frequently Asked Questions
What are the advantages of using microfluidic gradients for creating patterned tissues?
Microfluidic gradients offer several advantages for creating patterned tissues. They provide precise control over the microenvironment, allowing for the creation of complex and dynamic gradients. They also enable high-throughput screening and analysis, allowing for the study of multiple conditions simultaneously. Additionally, microfluidic devices are relatively inexpensive to fabricate and maintain, making them accessible to a wide range of researchers.
What are the potential applications of this patterned human neural tube model?
This model has a wide range of potential applications, including studying the mechanisms of neural tube development, identifying potential drug targets for treating neural tube defects, and developing new therapies for neurodevelopmental disorders. It can also be used to investigate the effects of environmental factors on neural tube development and to screen for potential teratogens.
What are the limitations of this model?
While this model offers significant advantages, it also has some limitations. It is a simplified representation of the complex in vivo environment, and it may not fully capture all aspects of neural tube development. Additionally, the model is still under development and requires further optimization and validation.






