De novo acute myeloid leukemia strom (DN-AML) is a complex and aggressive blood cancer characterized by the uncontrolled proliferation of abnormal myeloid cells in the bone marrow. While the genetic and molecular underpinnings of DN-AML are actively investigated, recent research highlights the crucial role of the stromal microenvironment in shaping disease progression and treatment response. This intricate network of cells and extracellular matrix components within the bone marrow provides a supportive niche for DN-AML cells, promoting their survival, proliferation, and resistance to therapy.
Understanding the intricate interactions between DN-AML cells and their stromal counterparts is critical for developing novel therapeutic strategies.
The stromal microenvironment, composed of fibroblasts, endothelial cells, mesenchymal stem cells, and other supporting cells, plays a multifaceted role in DN-AML pathogenesis. These stromal cells secrete growth factors and cytokines that influence the behavior of DN-AML cells, promoting their survival, proliferation, and drug resistance. Moreover, the stromal microenvironment provides a physical barrier, shielding DN-AML cells from cytotoxic agents and immune surveillance.
The interplay between DN-AML cells and their stromal counterparts involves complex signaling pathways and adhesion molecules, ultimately shaping the disease landscape and presenting challenges for effective treatment.
De Novo Acute Myeloid Leukemia (DN-AML)
De novo acute myeloid leukemia (DN-AML) is a type of cancer that affects the blood and bone marrow. It is characterized by the rapid and uncontrolled growth of abnormal myeloid cells, which are the cells that normally develop into mature blood cells, such as white blood cells, red blood cells, and platelets. In DN-AML, these cells fail to mature properly and accumulate in the bone marrow, crowding out healthy blood cells.
This leads to a deficiency in normal blood cells, resulting in a variety of symptoms, including fatigue, weakness, bleeding, and infections.
Incidence and Prevalence
DN-AML is a relatively rare but serious type of cancer. The incidence of DN-AML varies depending on age, geographic location, and other factors. It is most common in older adults, with the median age at diagnosis being around 65 years. The incidence of DN-AML is slightly higher in men than in women. Globally, DN-AML accounts for approximately 1% of all new cancer cases.
However, the incidence rates can vary significantly across different regions of the world. For example, in the United States, the incidence rate is estimated to be around 1.5 per 100,000 people per year.
Genetic and Molecular Mechanisms
The development of DN-AML is a complex process that involves a series of genetic and molecular alterations. These alterations can occur in a variety of genes, including those involved in cell growth, differentiation, and apoptosis (programmed cell death).
- Mutations in Genes Involved in Cell Growth and Differentiation:
Mutations in genes that regulate cell growth and differentiation, such as FLT3, NPM1, and CEBPA, can lead to uncontrolled cell proliferation and impaired differentiation of myeloid cells. These mutations are frequently found in DN-AML and can be associated with specific clinical features and treatment responses. - Mutations in Genes Involved in Apoptosis:
Mutations in genes involved in apoptosis, such as TP53 and RUNX1, can prevent the elimination of abnormal cells, contributing to the accumulation of leukemic cells. These mutations can also influence the response to chemotherapy and the overall prognosis of the disease. - Chromosomal Abnormalities:
Chromosomal abnormalities, such as translocations, inversions, and deletions, are common in DN-AML. These abnormalities can lead to the overexpression or underexpression of genes involved in leukemogenesis. For example, the translocation t(15;17) is associated with acute promyelocytic leukemia (APL), a subtype of AML. - Epigenetic Alterations:
Epigenetic alterations, such as changes in DNA methylation and histone modifications, can also play a role in DN-AML development. These alterations can affect gene expression patterns and contribute to the abnormal growth and differentiation of myeloid cells.
Stromal Microenvironment in DN-AML
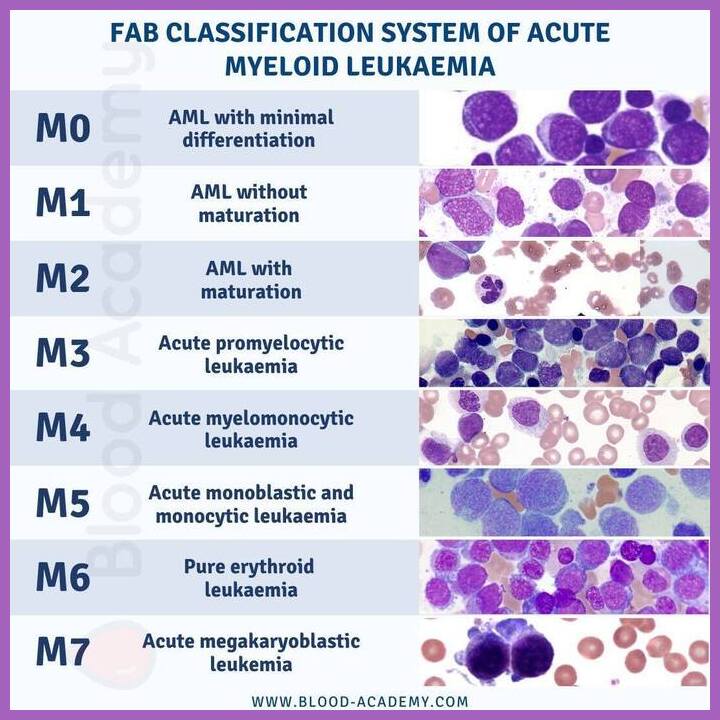
The stromal microenvironment in DN-AML is a complex and dynamic ecosystem that plays a crucial role in the pathogenesis, progression, and treatment response of the disease. It comprises a variety of non-hematopoietic cells, including fibroblasts, endothelial cells, mesenchymal stem cells (MSCs), and immune cells, that interact with AML cells and contribute to their survival, proliferation, and drug resistance.
Role of Stromal Cells in DN-AML Pathogenesis, De novo acute myeloid leukemia strom
Stromal cells create a supportive niche for AML cells by providing essential growth factors, cytokines, and adhesion molecules. They also contribute to the development of an immunosuppressive microenvironment that allows AML cells to evade immune surveillance.
- Fibroblasts are the most abundant stromal cell type and play a crucial role in the formation of the extracellular matrix (ECM), which provides structural support and regulates cell adhesion, migration, and signaling. In DN-AML, fibroblasts can produce factors that promote AML cell proliferation, such as vascular endothelial growth factor (VEGF) and fibroblast growth factor (FGF). They can also produce factors that inhibit apoptosis, such as interleukin-6 (IL-6) and transforming growth factor-beta (TGF-β).
- Endothelial cells line the blood vessels and play a critical role in angiogenesis, the formation of new blood vessels, which is essential for tumor growth and metastasis. In DN-AML, endothelial cells can be induced to produce factors that promote AML cell survival and proliferation, such as VEGF and platelet-derived growth factor (PDGF). They can also contribute to the formation of a pre-metastatic niche, which facilitates the spread of AML cells to other organs.
- Mesenchymal stem cells (MSCs) are multipotent cells that can differentiate into various cell types, including fibroblasts, chondrocytes, and osteoblasts. In DN-AML, MSCs can promote AML cell growth and survival by secreting growth factors, cytokines, and chemokines. They can also contribute to the development of drug resistance by providing a protective niche for AML cells and by suppressing the immune system.
Contribution of the Stromal Microenvironment to DN-AML Cell Survival, Proliferation, and Drug Resistance
The stromal microenvironment provides a protective niche for AML cells, shielding them from the cytotoxic effects of chemotherapy and promoting their survival and proliferation.
- Survival: Stromal cells secrete growth factors and cytokines that promote AML cell survival by activating signaling pathways that inhibit apoptosis. For example, stromal cells can produce IL-6, which activates the STAT3 pathway and promotes AML cell survival. They can also produce TGF-β, which inhibits apoptosis by activating the SMAD pathway.
- Proliferation: Stromal cells produce growth factors and cytokines that stimulate AML cell proliferation. For example, stromal cells can produce VEGF, which activates the VEGFR pathway and promotes AML cell proliferation. They can also produce FGF, which activates the FGFR pathway and promotes AML cell proliferation.
- Drug Resistance: The stromal microenvironment can contribute to drug resistance in DN-AML by several mechanisms. For example, stromal cells can produce factors that reduce the efficacy of chemotherapy drugs, such as MDR1, which pumps chemotherapy drugs out of AML cells. They can also provide a protective niche for AML cells, shielding them from the cytotoxic effects of chemotherapy drugs. Additionally, stromal cells can suppress the immune system, which can further enhance drug resistance.
Interactions Between DN-AML Cells and Stromal Cells
:max_bytes(150000):strip_icc()/GettyImages-842965240-00f76054677049bc84a11c6a5570fb69.jpg)
The intricate interplay between DN-AML cells and stromal cells within the bone marrow microenvironment plays a crucial role in shaping the disease progression and treatment response. This complex communication network involves a variety of signaling pathways, adhesion molecules, and growth factors, ultimately influencing the survival, proliferation, and chemoresistance of DN-AML cells.
Signaling Pathways Involved in Interactions
The communication between DN-AML cells and stromal cells is facilitated by various signaling pathways, including:
- Wnt Signaling: This pathway, often activated in DN-AML, promotes cell proliferation and survival. Stromal cells can secrete Wnt ligands, which bind to receptors on DN-AML cells, activating downstream signaling cascades that ultimately lead to the expression of genes involved in cell growth and survival.
- Hedgehog Signaling: This pathway is involved in cell fate determination and tissue patterning. Stromal cells can produce Hedgehog ligands, which can activate Hedgehog signaling in DN-AML cells, promoting their self-renewal and inhibiting differentiation.
- Notch Signaling: This pathway plays a role in cell fate decisions, proliferation, and survival. Stromal cells can express Notch ligands, which interact with receptors on DN-AML cells, triggering intracellular signaling cascades that influence DN-AML cell behavior.
- NF-κB Signaling: This pathway is involved in inflammation and immune responses. Stromal cells can secrete cytokines, such as TNF-α, which can activate NF-κB signaling in DN-AML cells, promoting their survival and resistance to apoptosis.
Adhesion Molecules Mediating Interactions
Cell-cell adhesion molecules play a vital role in establishing and maintaining the physical interactions between DN-AML cells and stromal cells. Key adhesion molecules involved include:
- Integrins: These transmembrane receptors mediate cell-cell and cell-extracellular matrix interactions. DN-AML cells express integrins, such as α4β1 and α5β1, which bind to stromal cell-derived ligands, such as fibronectin and VCAM-1, promoting cell adhesion and survival.
- Cadherins: These transmembrane proteins mediate cell-cell adhesion. DN-AML cells express cadherins, such as E-cadherin and N-cadherin, which can interact with stromal cell-expressed cadherins, contributing to the formation of a protective niche for DN-AML cells.
- Selectins: These transmembrane proteins mediate cell-cell interactions involving carbohydrate ligands. Stromal cells can express selectins, such as L-selectin, which can bind to carbohydrates on DN-AML cells, promoting their adhesion and interaction with the stromal microenvironment.
Growth Factors Involved in Interactions
Stromal cells produce and secrete various growth factors that can influence the behavior of DN-AML cells, including:
- VEGF (Vascular Endothelial Growth Factor): This growth factor promotes angiogenesis, the formation of new blood vessels. Stromal cells can secrete VEGF, which can stimulate angiogenesis in the bone marrow, providing DN-AML cells with a source of nutrients and oxygen.
- G-CSF (Granulocyte Colony-Stimulating Factor): This growth factor stimulates the production of neutrophils, a type of white blood cell. Stromal cells can secrete G-CSF, which can promote the proliferation and survival of DN-AML cells.
- IL-6 (Interleukin-6): This cytokine plays a role in inflammation and immune responses. Stromal cells can secrete IL-6, which can promote the proliferation and survival of DN-AML cells.
- SCF (Stem Cell Factor): This growth factor promotes the survival and proliferation of hematopoietic stem cells. Stromal cells can secrete SCF, which can support the growth and development of DN-AML cells.
Impact of Interactions on DN-AML Cell Behavior
The interactions between DN-AML cells and stromal cells have a profound impact on DN-AML cell behavior, including:
- Enhanced Survival and Proliferation: Stromal cells can provide a protective niche for DN-AML cells, promoting their survival and proliferation. This is achieved through the secretion of growth factors, cytokines, and the provision of adhesion molecules that support DN-AML cell growth and survival.
- Chemoresistance: The stromal microenvironment can protect DN-AML cells from the cytotoxic effects of chemotherapy. Stromal cells can create a physical barrier that limits the penetration of chemotherapy drugs, and they can also secrete factors that can induce drug resistance in DN-AML cells.
- Inhibition of Differentiation: Stromal cells can suppress the differentiation of DN-AML cells, promoting their self-renewal and maintaining their malignant phenotype. This can be achieved through the activation of signaling pathways, such as Hedgehog signaling, which promote self-renewal and inhibit differentiation.
- Angiogenesis: Stromal cells can contribute to the formation of new blood vessels in the bone marrow, providing DN-AML cells with a source of nutrients and oxygen. This can promote the growth and spread of DN-AML cells.
Therapeutic Implications of Stromal Microenvironment in DN-AML: De Novo Acute Myeloid Leukemia Strom
The intricate interplay between DN-AML cells and the stromal microenvironment presents a unique therapeutic opportunity. Targeting the stromal microenvironment holds immense promise for disrupting the protective shield that shields DN-AML cells from conventional therapies and for fostering a more favorable environment for treatment efficacy.
Strategies to Disrupt Interactions Between DN-AML Cells and Stromal Cells
The interactions between DN-AML cells and stromal cells are mediated by a complex network of signaling pathways and adhesion molecules. Disrupting these interactions can potentially sensitize DN-AML cells to chemotherapy and improve treatment outcomes.
- Blocking Adhesion Molecules: Antibodies targeting adhesion molecules, such as VCAM-1, ICAM-1, and CD44, have shown promise in preclinical studies. These antibodies can disrupt the physical attachment of DN-AML cells to the stromal niche, thereby reducing their protection from chemotherapy. For instance, a study published in
-Blood* demonstrated that an anti-VCAM-1 antibody significantly reduced the growth of AML cells in a xenograft model. - Targeting Signaling Pathways: Inhibiting signaling pathways that promote DN-AML cell survival and proliferation within the stromal microenvironment is another promising strategy. For example, targeting the Wnt/β-catenin pathway, which is known to be activated in the stromal niche and contributes to DN-AML cell growth, has shown encouraging results in preclinical studies. Studies have shown that inhibiting Wnt signaling can reduce the growth and survival of AML cells in vitro and in vivo.
- Modulating Stromal Cell Function: Modifying the function of stromal cells to create a less supportive environment for DN-AML cells is another therapeutic approach. For example, targeting stromal cells with agents that induce apoptosis or inhibit their production of pro-survival factors could reduce the protective effects of the microenvironment. A study in
-Cancer Research* showed that treatment with a small molecule inhibitor of the stromal cell-derived factor-1 (SDF-1) significantly reduced the growth of AML cells in a xenograft model.
Promising Therapeutic Agents that Modulate the Stromal Microenvironment in DN-AML
Several therapeutic agents are currently being investigated for their ability to modulate the stromal microenvironment in DN-AML. These agents target various aspects of the stromal microenvironment, aiming to disrupt the protective shield that shields DN-AML cells from conventional therapies.
- Anti-angiogenic Agents: These agents inhibit the formation of new blood vessels, which are essential for the growth and survival of DN-AML cells. Bevacizumab, a monoclonal antibody that targets vascular endothelial growth factor (VEGF), has shown promising results in clinical trials for AML patients.
- Small Molecule Inhibitors: These agents target specific signaling pathways involved in the interactions between DN-AML cells and stromal cells. For example, inhibitors of the Wnt/β-catenin pathway and the Hedgehog pathway are being investigated in preclinical studies.
- Immunotherapeutic Agents: These agents aim to stimulate the immune system to attack DN-AML cells. For example, chimeric antigen receptor (CAR) T-cell therapy, which involves genetically modifying T cells to target specific antigens on DN-AML cells, is showing promising results in clinical trials.
Future Directions in Research
While significant progress has been made in understanding the role of the stromal microenvironment in DN-AML, several knowledge gaps and research challenges remain. Addressing these gaps will be crucial for developing novel therapeutic strategies that effectively target the complex interplay between DN-AML cells and the stromal microenvironment.
Identifying Specific Stromal Subtypes and Their Functional Roles
The stromal microenvironment in DN-AML is heterogeneous, comprising various cell types with distinct functions. Current research has focused on identifying and characterizing the major stromal cell populations involved in DN-AML pathogenesis. However, further research is needed to:
- Identify and characterize novel stromal cell subtypes and their specific roles in DN-AML progression.
- Investigate the spatial distribution and interactions of different stromal cell subtypes within the bone marrow microenvironment.
- Determine the specific molecular mechanisms by which different stromal cell subtypes contribute to DN-AML cell survival, proliferation, and drug resistance.
Understanding the precise functions of specific stromal subtypes will be crucial for developing targeted therapies that selectively modulate the activities of these cells.
Developing Novel Therapeutic Strategies Targeting the Stromal Microenvironment
Current therapeutic approaches for DN-AML primarily focus on targeting the malignant cells themselves. However, the stromal microenvironment provides a protective niche for DN-AML cells, contributing to their resistance to conventional therapies. Therefore, novel therapeutic strategies that target the stromal microenvironment hold great promise for improving DN-AML treatment outcomes.
- Stromal-Targeted Therapies: Development of drugs that specifically target stromal cells involved in DN-AML pathogenesis, such as inhibitors of signaling pathways that promote DN-AML cell survival or proliferation.
- Modulation of Stromal-Leukemia Cell Interactions: Strategies to disrupt the interactions between DN-AML cells and stromal cells, such as blocking adhesion molecules or disrupting the production of growth factors that support DN-AML cell growth.
- Immunotherapy Targeting the Stromal Microenvironment: Development of immune-based therapies that target stromal cells or their interactions with DN-AML cells, such as CAR T-cell therapies engineered to recognize stromal-specific antigens or checkpoint inhibitors that enhance the immune response against stromal cells.
These novel therapeutic approaches have the potential to overcome drug resistance, enhance treatment efficacy, and improve patient outcomes.
Investigating the Role of the Stromal Microenvironment in DN-AML Drug Resistance
DN-AML cells often develop resistance to conventional chemotherapies, leading to treatment failure. The stromal microenvironment plays a significant role in promoting drug resistance by:
- Providing a protective niche that shields DN-AML cells from cytotoxic agents.
- Activating drug efflux pumps that pump out chemotherapeutic drugs from DN-AML cells.
- Producing factors that induce drug resistance in DN-AML cells.
Further research is needed to:
- Identify the specific mechanisms by which the stromal microenvironment contributes to drug resistance in DN-AML.
- Develop strategies to overcome drug resistance by targeting the stromal microenvironment.
This research will be crucial for developing more effective treatment strategies for DN-AML that overcome drug resistance and improve patient survival.
Integrating Multi-Omics Approaches to Understand the Stromal Microenvironment
The stromal microenvironment is a complex and dynamic system involving multiple cell types and intricate interactions. To fully understand the role of the stromal microenvironment in DN-AML, it is essential to integrate multi-omics approaches, including:
- Genomics: Sequencing the genomes of stromal cells to identify genetic alterations that contribute to DN-AML pathogenesis.
- Transcriptomics: Analyzing the gene expression profiles of stromal cells to identify genes involved in DN-AML cell support and drug resistance.
- Proteomics: Studying the protein expression profiles of stromal cells to identify proteins that mediate interactions with DN-AML cells.
- Metabolomics: Investigating the metabolic profiles of stromal cells to understand their contribution to DN-AML cell metabolism and drug resistance.
By integrating these multi-omics approaches, researchers can gain a comprehensive understanding of the molecular mechanisms underlying the interactions between DN-AML cells and the stromal microenvironment.
Developing Patient-Derived Models to Study the Stromal Microenvironment
Preclinical studies using animal models have provided valuable insights into the role of the stromal microenvironment in DN-AML. However, these models may not fully recapitulate the complexity of the human microenvironment.
- Developing patient-derived xenograft (PDX) models that include both DN-AML cells and the patient’s own stromal cells.
- Utilizing organ-on-a-chip technology to create microfluidic devices that mimic the bone marrow microenvironment, allowing for the study of DN-AML cells and stromal cells in a more physiologically relevant setting.
These patient-derived models will provide more accurate and relevant insights into the role of the stromal microenvironment in DN-AML pathogenesis and drug resistance, facilitating the development of more effective therapeutic strategies.
Targeting the stromal microenvironment in DN-AML offers a promising avenue for therapeutic intervention. By disrupting the interactions between DN-AML cells and stromal cells, researchers aim to disrupt the supportive niche that fosters disease progression. This approach involves exploring strategies to block the signaling pathways involved in these interactions, inhibit the production of growth factors and cytokines by stromal cells, and enhance the immune system’s ability to recognize and eliminate DN-AML cells.
The future of DN-AML treatment holds the potential for novel therapies that effectively modulate the stromal microenvironment, paving the way for improved outcomes and a better understanding of this complex disease.
Q&A
What are the main types of stromal cells involved in DN-AML?
The main types of stromal cells involved in DN-AML include fibroblasts, endothelial cells, and mesenchymal stem cells. These cells contribute to the microenvironment by providing structural support, producing growth factors, and influencing the behavior of DN-AML cells.
How does the stromal microenvironment contribute to drug resistance in DN-AML?
The stromal microenvironment can contribute to drug resistance in DN-AML by creating a protective barrier around DN-AML cells, reducing the penetration of chemotherapeutic agents. Stromal cells can also produce factors that promote drug efflux, effectively pumping chemotherapy drugs out of DN-AML cells, limiting their efficacy.
What are some potential therapeutic targets within the stromal microenvironment?
Potential therapeutic targets within the stromal microenvironment include signaling pathways involved in interactions between DN-AML cells and stromal cells, the production of growth factors and cytokines by stromal cells, and the expression of adhesion molecules that mediate cell-cell interactions.






