How does the heart contract fee wall apex to base – How does the heart contract from apex to base? This seemingly simple question unveils a complex and fascinating orchestration of electrical signals and mechanical actions within the heart. Understanding this process requires exploring the intricate interplay between the heart’s conduction system, the mechanics of myocardial cell contraction, and the hemodynamics of blood flow. This journey will reveal how a coordinated sequence of events, starting with the sinoatrial node’s electrical impulse and culminating in the forceful ejection of blood, ensures the efficient pumping action of the heart.
From the initial electrical depolarization that sweeps through the heart muscle, initiating contraction at the apex, to the final squeezing action that propels blood towards the base and out into the circulatory system, every step is crucial. We will examine the roles of key structures like the atrioventricular node, the Purkinje fibers, and the calcium ions essential for muscle contraction.
We’ll also consider the impact of the heart’s unique anatomical orientation on the directionality of its contraction.
Cardiac Conduction System and its Role

The heart, a tireless pump, doesn’t rely on conscious effort to beat. Instead, a specialized network of cells, the cardiac conduction system, orchestrates its rhythmic contractions. This intricate system ensures a coordinated sequence of atrial and ventricular contractions, propelling blood efficiently throughout the body. Think of it as the heart’s own internal electrical wiring, ensuring every beat is precise and powerful.The pathway of the electrical impulse begins at the sinoatrial (SA) node, often called the heart’s natural pacemaker.
Located in the right atrium, the SA node spontaneously generates electrical impulses at a regular rate, initiating each heartbeat. This impulse spreads rapidly through the atrial myocardium, causing atrial contraction. From the SA node, the impulse travels to the atrioventricular (AV) node, a crucial relay station situated between the atria and ventricles.
Atrioventricular Node Delay
The AV node plays a vital role in coordinating atrial and ventricular contraction. It introduces a slight delay in the conduction of the electrical impulse. This delay is not a malfunction; it’s essential to allow the atria to fully contract and empty their blood into the ventricles before ventricular contraction begins. Without this delay, the ventricles would contract prematurely, reducing the efficiency of blood pumping.
The delay is achieved through slower conduction velocity in the AV node compared to other parts of the conduction system.
Impulse Propagation Through the Ventricles, How does the heart contract fee wall apex to base
After the AV node, the electrical impulse travels down the bundle of His, a specialized pathway that penetrates the fibrous tissue separating the atria and ventricles. The bundle of His then branches into the right and left bundle branches, carrying the impulse to the respective ventricles. These branches further divide into a network of Purkinje fibers, which distribute the impulse throughout the ventricular myocardium, triggering a powerful and coordinated ventricular contraction.
This synchronized contraction efficiently ejects blood into the pulmonary artery and aorta.
Conduction Speed Variations
The speed of impulse conduction varies across different parts of the cardiac conduction system. The SA node and atrial muscle have relatively fast conduction speeds, ensuring rapid atrial depolarization. The AV node, as mentioned earlier, exhibits significantly slower conduction to allow for the atrioventricular delay. The bundle of His, bundle branches, and Purkinje fibers conduct the impulse at very high speeds, facilitating rapid and synchronized ventricular depolarization.
These variations in conduction speed are crucial for the precisely timed sequence of atrial and ventricular contractions, essential for efficient blood circulation. The fast conduction in the Purkinje fibers, for example, ensures that the ventricles contract almost simultaneously, maximizing the ejection of blood.
Myocardial Cell Contraction Mechanics: How Does The Heart Contract Fee Wall Apex To Base
The rhythmic beating of our hearts, a testament to the coordinated actions of billions of cells, hinges on the intricate dance of excitation-contraction coupling within individual cardiomyocytes. This process transforms electrical signals into the powerful mechanical contractions that propel blood throughout our bodies. Let’s delve into the molecular mechanisms that orchestrate this vital process.
The heart’s ability to contract relies on a fascinating interplay between electrical excitation and the contractile machinery of the muscle cells. This interplay, known as excitation-contraction coupling, is a finely tuned process involving the influx of calcium ions, the interaction of proteins within the sarcomeres, and the sliding filament mechanism that ultimately shortens the muscle fibers.
Excitation-Contraction Coupling in Cardiac Muscle Cells
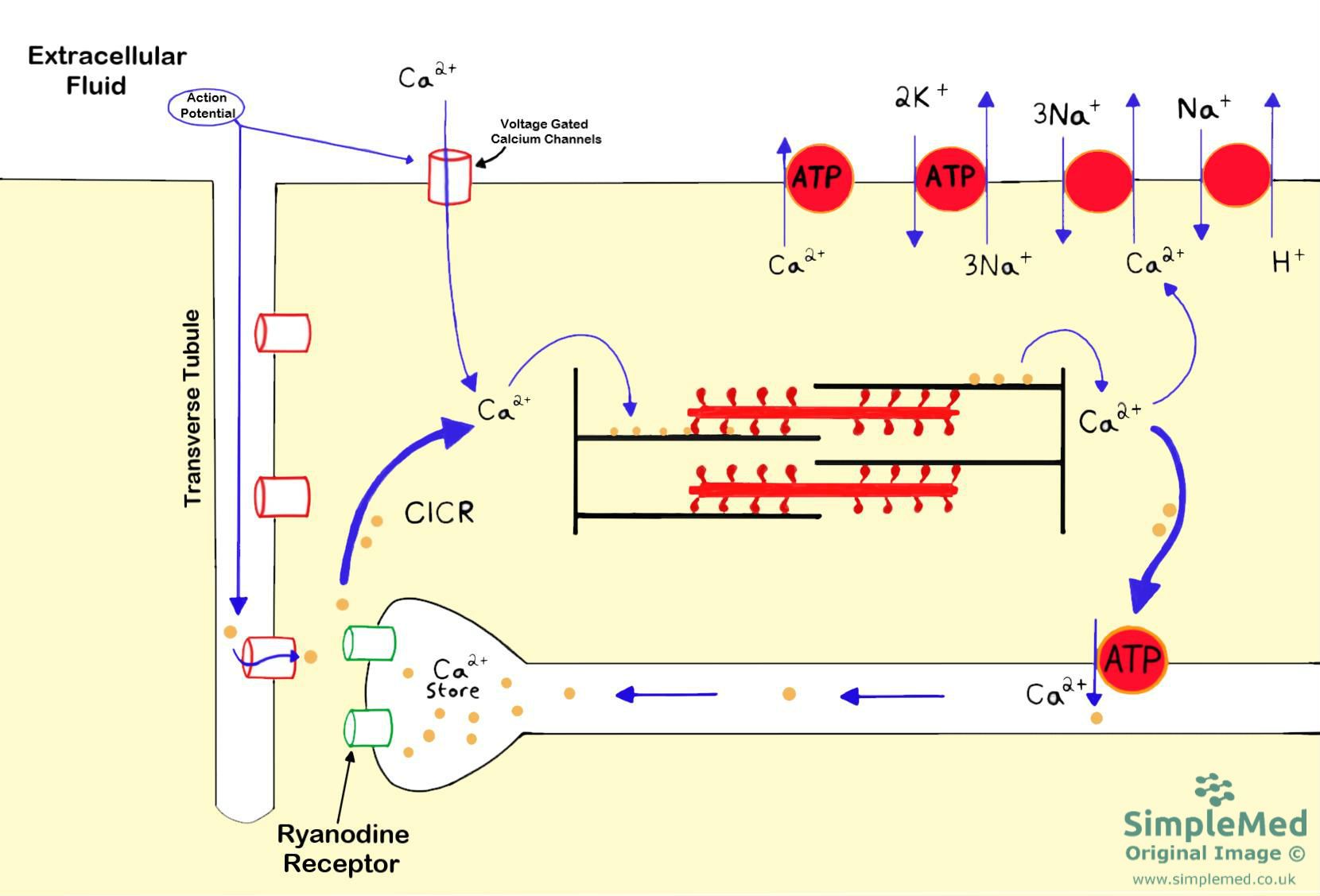
The process begins with the arrival of an action potential at the sarcolemma, the cardiac muscle cell membrane. This depolarization opens voltage-gated L-type calcium channels, allowing a small influx of calcium ions (Ca2+) into the cell. This initial Ca2+ influx, though small, triggers a much larger release of Ca2+ from the sarcoplasmic reticulum (SR), an intracellular calcium store. This process, known as calcium-induced calcium release (CICR), amplifies the initial signal, ensuring a robust intracellular Ca2+ concentration increase.
The increased cytosolic Ca2+ concentration is crucial because it interacts with the contractile proteins within the sarcomeres. Specifically, Ca2+ binds to troponin C, a protein complex situated on the thin filaments (actin filaments). This binding causes a conformational change in troponin, which in turn moves tropomyosin, another protein on the thin filaments, away from the myosin-binding sites on actin.
The Sliding Filament Theory in Cardiomyocytes
With the myosin-binding sites on actin now exposed, the stage is set for the sliding filament mechanism. Myosin, a motor protein located on the thick filaments, possesses ATPase activity. Hydrolysis of ATP provides the energy for myosin heads to bind to actin, forming cross-bridges. The myosin heads then undergo a conformational change, pulling the thin filaments towards the center of the sarcomere, causing the sarcomere to shorten.
This process repeats as long as Ca2+ remains bound to troponin C. The cyclical binding, pivoting, and detachment of myosin heads along the actin filaments generate the force of contraction. The coordinated shortening of numerous sarcomeres within a cardiomyocyte results in the overall contraction of the muscle cell. The continuous cycling of myosin heads along the actin filaments requires a continuous supply of ATP.
Sarcomere Shortening: A Step-by-Step Description
- Action potential arrives at the sarcolemma.
- L-type calcium channels open, allowing Ca2+ influx.
- Calcium-induced calcium release (CICR) from the sarcoplasmic reticulum.
- Increased cytosolic Ca2+ binds to troponin C.
- Tropomyosin moves, exposing myosin-binding sites on actin.
- Myosin heads bind to actin, forming cross-bridges.
- ATP hydrolysis powers the myosin power stroke, pulling thin filaments towards the center of the sarcomere.
- Myosin heads detach from actin; the cycle repeats as long as Ca2+ is bound to troponin C.
- Sarcomeres shorten, leading to cardiomyocyte contraction.
- Ca2+ is actively pumped back into the SR and extracellular space, leading to relaxation.
The Heart’s Electrical Activity and its Relation to Contraction
The rhythmic beating of our heart, a seemingly effortless process, is orchestrated by a complex interplay of electrical and mechanical events. We’ve already explored how the heart’s structure facilitates its efficient pumping action, and the intricate pathways of the cardiac conduction system. Now, let’s delve into how electrical signals initiate and coordinate the mechanical contractions that propel blood throughout the body.
This intricate dance between electricity and mechanics is what keeps us alive.The electrocardiogram (ECG) provides a window into the heart’s electrical activity. It’s a non-invasive recording of the electrical potentials generated by the heart as it contracts and relaxes. By analyzing the ECG waves, we can gain valuable insights into the timing and sequence of cardiac events, and identify potential problems in the heart’s electrical conduction system.
ECG Waves and Phases of Contraction
The ECG displays characteristic waves that correspond to specific phases of the cardiac cycle. The following table illustrates the correlation between these waves and the mechanical events of contraction.
| ECG Wave | Cardiac Cycle Phase | Mechanical Event | Description |
|---|---|---|---|
| P wave | Atrial depolarization | Atrial contraction | Represents the electrical activation of the atria, leading to their contraction and emptying of blood into the ventricles. |
| QRS complex | Ventricular depolarization | Ventricular contraction | Represents the rapid depolarization of the ventricles, initiating their powerful contraction and ejection of blood into the pulmonary artery and aorta. The Q, R, and S waves represent different phases of this depolarization. |
| T wave | Ventricular repolarization | Ventricular relaxation | Represents the recovery phase of the ventricles, as they repolarize and prepare for the next contraction. |
Depolarization Wave Propagation and its Effect on Contraction
The depolarization wave, originating from the sinoatrial (SA) node, the heart’s natural pacemaker, spreads systematically through the heart muscle. This organized spread ensures that the atria contract first, followed by the ventricles, a crucial sequence for efficient blood ejection. The wave travels rapidly through the specialized conduction pathways, ensuring a coordinated contraction from apex to base. This coordinated contraction is essential for efficient blood ejection from the heart.
The atria contract first, pushing blood into the ventricles. Then, the ventricles contract, powerfully propelling blood into the pulmonary artery and aorta. The delay introduced by the atrioventricular (AV) node allows the atria to fully empty before the ventricles contract. This precise timing is critical for maximizing cardiac output.
Sequence of Events from Electrical Stimulation to Mechanical Contraction
The following flowchart depicts the sequence of events within a single cardiomyocyte, from electrical stimulation to mechanical contraction. Flowchart:
1. Electrical Stimulation
The depolarization wave reaches the cardiomyocyte’s cell membrane.
2. Membrane Depolarization
Sodium channels open, causing rapid influx of sodium ions and a change in membrane potential.
3. Calcium Influx
Depolarization triggers the opening of L-type calcium channels, allowing calcium ions to enter the cell.
4. Calcium-Induced Calcium Release (CICR)
The influx of calcium triggers the release of even more calcium from the sarcoplasmic reticulum (SR), a specialized intracellular calcium store.
5. Increased Cytosolic Calcium
The combined influx and release of calcium leads to a significant increase in cytosolic calcium concentration.
6. Cross-Bridge Cycling
The increased calcium binds to troponin C, initiating a series of events that lead to the interaction of actin and myosin filaments, resulting in muscle contraction.
7. Mechanical Contraction
The interaction of actin and myosin filaments generates force, leading to shortening of the sarcomere and ultimately, the contraction of the cardiomyocyte.
8. Repolarization
Potassium channels open, allowing potassium ions to exit the cell, restoring the resting membrane potential.
9. Calcium Removal
Calcium is actively pumped back into the SR and out of the cell, leading to muscle relaxation.
Hemodynamics and the Direction of Contraction

The heart’s ingenious design, with its unique orientation and sophisticated contraction sequence, is crucial for efficient blood ejection. Understanding how the heart’s structure influences its function reveals a remarkable interplay between anatomy and hemodynamics. The coordinated contraction, progressing from apex to base, isn’t simply a random event; it’s a precisely orchestrated mechanism designed to maximize blood flow and minimize energy expenditure.The heart’s orientation, with the apex pointing downwards and slightly to the left, plays a pivotal role in this process.
Blood entering the ventricles fills the chambers passively. As the ventricles contract, this base-to-apex sequence acts like a powerful squeeze, propelling blood upwards towards the semilunar valves and out into the pulmonary artery and aorta. Imagine squeezing a tube of toothpaste from the bottom – the pressure efficiently pushes the contents out the top. This is analogous to the heart’s action; the coordinated contraction ensures that blood is efficiently ejected, rather than simply being pushed haphazardly in all directions.
Ventricular Pressure Changes During Systole and Their Relation to Contraction Direction
During ventricular systole, pressure rises dramatically. This increase isn’t uniform throughout the ventricle. The pressure initially builds more significantly in the apex, initiating the ejection process. As the contraction progresses towards the base, the pressure continues to rise, pushing blood against the semilunar valves. This pressure gradient, established by the sequential contraction, ensures unidirectional blood flow and prevents backflow.
The pressure wave, originating from the apex and culminating at the base, efficiently empties the ventricles. This efficient emptying minimizes residual blood volume in the ventricles after systole, maximizing stroke volume and cardiac output. Consider the pressure changes as a wave propagating through the ventricle, maximizing the efficiency of blood ejection. The pressure at the base is significantly higher than at the apex at the end of systole, demonstrating the effectiveness of the apex-to-base contraction.
Physiological Mechanisms Ensuring Efficient Ejection of Blood from the Ventricles
Several physiological mechanisms contribute to the efficient ejection of blood. The helical arrangement of cardiac muscle fibers within the ventricles is crucial. These fibers are not arranged in simple parallel lines; instead, they spiral around the ventricles, resembling a tightly wound spring. This arrangement enhances the “wringing” action of the contraction, twisting and squeezing the blood towards the outflow tracts.
Furthermore, the papillary muscles and chordae tendineae prevent the atrioventricular valves from inverting during ventricular contraction, ensuring unidirectional flow towards the aorta and pulmonary artery. The coordinated timing of the contraction, guided by the cardiac conduction system, is essential. The sequential activation of cardiomyocytes from apex to base ensures a smooth and efficient ejection, preventing turbulent flow and maximizing stroke volume.
This precise choreography of muscle contraction and valve function is essential for maintaining effective circulatory function.
Variations in Contraction and Clinical Implications
The heart’s rhythmic contractions, a beautifully orchestrated dance of electrical and mechanical events, are essential for life. However, this finely tuned system is susceptible to disruptions, leading to a range of conditions that compromise its efficiency and, ultimately, the body’s ability to thrive. Understanding these variations in contraction and their clinical implications is crucial for diagnosis and treatment.The coordinated contraction of the heart relies on a precise sequence of electrical activation, originating in the sinoatrial (SA) node and propagating through the conduction system.
Any interference with this intricate process can result in arrhythmias – irregular heartbeats – or conduction blocks – interruptions in the transmission of electrical impulses. These disruptions profoundly impact the heart’s ability to pump blood effectively.
Arrhythmias and Conduction Blocks: Causes and Mechanisms
Arrhythmias encompass a wide spectrum of disorders, ranging from occasional premature beats to life-threatening tachycardias (rapid heart rates) or bradycardias (slow heart rates). Causes are diverse and include electrolyte imbalances (like low potassium or magnesium), structural heart disease (such as coronary artery disease or cardiomyopathy), infections (myocarditis), and genetic factors. Conduction blocks, on the other hand, arise from disruptions in the pathways of the conduction system, often due to damage from heart attacks, fibrosis (scarring), or congenital abnormalities.
For example, a bundle branch block occurs when the electrical impulse is delayed or blocked in one of the bundle branches, leading to asynchronous ventricular contraction. Atrioventricular (AV) blocks, stemming from impaired conduction through the AV node, can range in severity, from mild first-degree blocks to complete heart blocks (third-degree) requiring pacemakers.
Consequences of Impaired Coordinated Contraction
Disruptions in the normal sequence of contraction significantly impact cardiac output – the volume of blood pumped by the heart per minute. In arrhythmias like atrial fibrillation, the chaotic electrical activity leads to inefficient atrial contraction, reducing the amount of blood reaching the ventricles and lowering stroke volume. Similarly, in conduction blocks, the delay or interruption in ventricular activation reduces the force and coordination of ventricular contraction, also diminishing cardiac output.
The consequences are far-reaching, manifesting as symptoms such as shortness of breath (dyspnea), chest pain (angina), dizziness, syncope (fainting), and even sudden cardiac death. The reduced cardiac output compromises the body’s ability to deliver oxygen and nutrients to tissues, potentially leading to organ damage and circulatory shock.
Comparison of Different Heart Block Effects on Ventricular Contraction
Different types of heart blocks exhibit varying effects on the sequence of ventricular contraction. First-degree AV block involves a prolonged PR interval (the time between atrial and ventricular activation) but still maintains a consistent 1:1 relationship between atrial and ventricular beats. Second-degree AV blocks are characterized by intermittent failure of some atrial impulses to conduct to the ventricles, resulting in dropped beats.
This can be further classified into Mobitz type I and Mobitz type II, each with distinct patterns of conduction. Complete heart block (third-degree) represents a complete dissociation between atrial and ventricular activity, with the ventricles beating independently at a slower rate (usually driven by the bundle of His or Purkinje fibers). This leads to significantly reduced cardiac output and requires immediate intervention, often with a pacemaker to restore coordinated ventricular contraction.
The varying degrees of impairment in conduction result in a spectrum of clinical manifestations, ranging from minimal symptoms to life-threatening consequences.
Illustrative Representation of Contraction Sequence
Imagine a three-dimensional model of the heart, a complex, muscular pump. Understanding its contraction isn’t simply about a squeezing action; it’s a coordinated, spiraling movement that efficiently ejects blood. This model allows us to visualize the intricate choreography of the heart’s muscle fibers during systole.To truly appreciate the heart’s contractile process, we must move beyond the simplistic idea of a uniform squeeze.
Instead, picture the heart’s muscle fibers arranged in a helical pattern, spiraling from apex to base. This arrangement is crucial for the heart’s unique twisting and wringing motion during contraction.
Three-Dimensional Model of Contraction Wave Propagation
Our three-dimensional model begins with the electrical impulse originating in the sinoatrial (SA) node. This impulse spreads rapidly through the atria, causing atrial contraction. However, our focus here is on the ventricular contraction. The impulse then travels down the conduction system, reaching the apex of the heart first. The apex, the bottom tip of the heart, initiates contraction.
The model vividly displays how this contraction doesn’t simply push blood upwards; it begins a twisting motion. As the wave of contraction moves superiorly towards the base, the heart effectively “wrings” the blood out, like squeezing a wet towel. The helical arrangement of the muscle fibers is crucial to this wringing action; the fibers shorten and twist simultaneously, maximizing the efficiency of blood ejection.
The model allows us to see the progression of this twist, demonstrating how the apex rotates slightly clockwise (in a normal heart) before the base follows suit. The coordinated nature of this twisting and wringing action is paramount to efficient cardiac output.
Ventricular Shape and Volume Changes During Systole
This illustration depicts the dynamic changes in ventricular shape and volume throughout systole. At the beginning of systole, the ventricles are relatively relaxed and filled with blood. As the contraction wave initiates at the apex, the ventricular volume begins to decrease. The model clearly shows how the apex initially contracts, reducing its chamber volume. Simultaneously, the apex’s rotation begins the twisting motion.
As the contraction wave progresses towards the base, the ventricles take on a more elongated, narrower shape. This shape change is directly related to the helical arrangement of the myocardial fibers. The model further illustrates the continued reduction in ventricular volume as the base contracts, completing the “wringing” process. The final stage depicts the ventricles at the end of systole, significantly reduced in volume and ready for diastole (relaxation) and refilling.
The coordinated contraction from apex to base is visually striking, highlighting the efficiency of this mechanism for blood ejection. The model effectively communicates how the change in ventricular shape and volume is not simply a reduction in size, but a dynamic process driven by the spiraling contraction of the myocardium.
In conclusion, the heart’s contraction, progressing from apex to base, is a marvel of coordinated biological engineering. The precise sequence of electrical activation and subsequent mechanical contraction, influenced by the heart’s structure and hemodynamics, ensures efficient blood ejection and systemic circulation. Understanding this intricate process is vital for appreciating the complexities of cardiovascular health and for diagnosing and treating various cardiac conditions that disrupt this finely tuned mechanism.
The precise interplay of electrical signals, cellular mechanics, and fluid dynamics creates a system that is both powerful and remarkably efficient.
Detailed FAQs
What happens if the apex-to-base contraction sequence is disrupted?
Disruptions can lead to reduced cardiac output, heart failure, and potentially life-threatening arrhythmias. The specific consequences depend on the nature and severity of the disruption.
How does the heart’s twisting motion contribute to efficient ejection?
The twisting motion, or torsion, helps to wring blood out of the ventricles, improving ejection efficiency and maximizing stroke volume.
Can you explain the role of the Purkinje fibers in this process?
Purkinje fibers rapidly conduct the electrical impulse throughout the ventricular myocardium, ensuring a coordinated and simultaneous contraction of the ventricular walls.
What is the significance of the delay at the AV node?
The delay allows the atria to fully contract and empty their contents into the ventricles before ventricular contraction begins, optimizing cardiac output.






