Does stroma regenerate? This question delves into the fascinating realm of tissue repair, where the supporting framework of organs and tissues plays a critical role. Stroma, the intricate network of cells and extracellular matrix, provides structural support, facilitates communication, and influences the behavior of surrounding cells. Its ability to regenerate is crucial for maintaining tissue integrity and restoring function after injury or disease.
This exploration delves into the complex mechanisms governing stroma regeneration, examining the factors that promote and hinder this process. We will explore the diverse regenerative capacities of different stromal tissues, investigate the clinical applications of stroma regeneration, and discuss the promising future directions in this field.
Introduction to Stroma
Stroma is the supporting framework of an organ or tissue, providing structural support and a microenvironment for the functional cells within it. It is a complex network of cells and extracellular matrix (ECM) that plays a vital role in maintaining tissue integrity, regulating cell behavior, and facilitating communication between cells.Stroma is not just a passive scaffolding but actively participates in the development, function, and regeneration of tissues and organs.
It provides physical support, regulates cell growth and differentiation, and plays a role in immune responses and wound healing.
Composition of Stroma
The composition of stroma varies depending on the organ or tissue, but it generally consists of two main components:
- Cellular components: These include various cell types, such as fibroblasts, endothelial cells, pericytes, smooth muscle cells, and immune cells. Each cell type contributes to the specific functions of the stroma.
- Extracellular matrix (ECM): This is a complex network of proteins and other molecules that provide structural support, regulate cell behavior, and facilitate communication between cells. The ECM is composed of fibers, such as collagen and elastin, and ground substance, which includes proteoglycans, glycoproteins, and water.
Types of Stroma
Different tissues and organs have specialized stromal components adapted to their unique functions. Here are some examples:
- Connective tissue stroma: This is the most common type of stroma, found in various organs and tissues. It provides structural support, binds tissues together, and facilitates the passage of nutrients and waste products. Examples include the stroma of the skin, muscles, and bones.
- Vascular stroma: This type of stroma is responsible for supplying blood and oxygen to the functional cells of an organ or tissue. It consists of blood vessels, lymphatics, and associated cells. Examples include the stroma of the liver, kidneys, and brain.
- Immune stroma: This type of stroma plays a crucial role in immune responses. It contains immune cells, such as lymphocytes, macrophages, and dendritic cells, which help to protect the body from infection and disease. Examples include the stroma of the lymph nodes, spleen, and bone marrow.
Functions of Stroma
Stroma performs various essential functions in tissues and organs:
- Structural support: Stroma provides a framework that helps maintain the shape and integrity of tissues and organs. The ECM provides tensile strength and flexibility, allowing tissues to withstand mechanical stress.
- Regulation of cell behavior: The ECM and stromal cells interact with functional cells, influencing their growth, differentiation, migration, and survival. This interaction is crucial for tissue development, regeneration, and repair.
- Tissue homeostasis: Stroma helps maintain the balance of cell populations and tissue function. It regulates the production and degradation of ECM components, ensuring tissue integrity and stability.
- Immune responses: Immune stroma provides a platform for immune cells to interact and mount appropriate responses to pathogens and foreign substances. It facilitates the recruitment and activation of immune cells, helping to control inflammation and protect the body from disease.
- Wound healing: Stroma plays a critical role in wound healing. It provides a scaffold for new tissue formation, promotes cell migration and proliferation, and regulates the production of growth factors and cytokines involved in repair processes.
Stroma Regeneration
Stroma regeneration is a complex process that involves the coordinated action of various cell types and signaling pathways to repair and restore damaged or lost stromal tissue. This intricate process is essential for maintaining tissue homeostasis and function, ensuring that tissues can recover from injury or disease.
Mechanisms of Stroma Regeneration
Stroma regeneration involves a series of orchestrated events that restore the structural integrity and functional capacity of damaged stromal tissue. The process encompasses three key mechanisms: cell proliferation, differentiation, and extracellular matrix remodeling.
- Cell Proliferation: The first step in stroma regeneration involves the proliferation of stromal cells, such as fibroblasts, endothelial cells, and pericytes. These cells are responsible for synthesizing and depositing the extracellular matrix, providing structural support and facilitating tissue repair. Growth factors, such as platelet-derived growth factor (PDGF) and fibroblast growth factor (FGF), play a crucial role in stimulating cell proliferation and migration to the site of injury.
- Differentiation: Once stromal cells have proliferated, they differentiate into specialized cell types that are required for tissue repair. For example, fibroblasts differentiate into myofibroblasts, which are responsible for wound contraction and scar formation. This differentiation process is regulated by a complex interplay of signaling pathways and transcription factors.
- Extracellular Matrix Remodeling: The extracellular matrix (ECM) provides structural support and organization to tissues. During stroma regeneration, the ECM undergoes significant remodeling, with the degradation of damaged components and the deposition of new ECM components. This process is facilitated by matrix metalloproteinases (MMPs), which are enzymes that degrade ECM components, and by ECM-producing cells, such as fibroblasts, which synthesize and deposit new ECM components.
Role of Signaling Pathways and Growth Factors
Signaling pathways and growth factors play a critical role in orchestrating stroma regeneration. These molecules act as messengers, relaying information about tissue damage and initiating the necessary cellular responses to repair the injured tissue.
- Growth Factors: Growth factors, such as PDGF, FGF, transforming growth factor-beta (TGF-β), and vascular endothelial growth factor (VEGF), stimulate cell proliferation, migration, differentiation, and ECM remodeling. They bind to specific receptors on target cells, triggering intracellular signaling cascades that regulate gene expression and cellular behavior.
- Signaling Pathways: Signaling pathways, such as the MAPK, PI3K/AKT, and Wnt pathways, are involved in regulating various aspects of stroma regeneration. These pathways are activated by growth factors and other signaling molecules, leading to changes in gene expression and cellular function. For example, the MAPK pathway promotes cell proliferation and survival, while the Wnt pathway regulates cell differentiation and ECM production.
Regenerative Capacity of Different Stromal Tissues
The regenerative capacity of different stromal tissues varies depending on factors such as tissue type, age, and the extent of injury. Some tissues, such as the liver and bone marrow, have a high regenerative capacity, while others, such as the heart and brain, have limited regenerative potential.
- High Regenerative Capacity: Tissues like the liver and bone marrow possess a high regenerative capacity due to the presence of stem cells and progenitor cells that can differentiate into various cell types. For example, hepatocytes, the main cell type in the liver, have a remarkable ability to regenerate after injury.
- Limited Regenerative Capacity: Tissues such as the heart and brain have limited regenerative potential because they contain a smaller pool of stem cells and progenitor cells. Furthermore, the complex architecture and specialized function of these tissues make regeneration challenging.
Factors Influencing Stroma Regeneration
Stroma regeneration is a complex process influenced by a variety of factors, both promoting and inhibiting its progression. Understanding these factors is crucial for developing strategies to enhance tissue repair and regeneration.
Factors Promoting Stroma Regeneration
Growth factors, cytokines, and biomaterials play a significant role in promoting stroma regeneration. These factors act as signaling molecules, stimulating cell proliferation, differentiation, and migration, ultimately leading to the formation of new stromal tissue.
- Growth Factors: Growth factors are proteins that bind to specific receptors on cells, triggering a cascade of intracellular signaling events that promote cell growth and division. Examples of growth factors that promote stroma regeneration include fibroblast growth factors (FGFs), transforming growth factor-beta (TGF-β), and vascular endothelial growth factor (VEGF).
- Cytokines: Cytokines are small proteins that act as signaling molecules between cells, regulating various cellular processes, including inflammation, immune responses, and tissue repair.
Examples of cytokines that promote stroma regeneration include interleukin-10 (IL-10), which suppresses inflammation, and tumor necrosis factor-alpha (TNF-α), which stimulates fibroblast proliferation and collagen production.
- Biomaterials: Biomaterials are synthetic or natural materials used to replace or enhance damaged tissues. Biomaterials can act as scaffolds for cell growth and differentiation, providing structural support and promoting the formation of new stromal tissue.
Examples of biomaterials used in stroma regeneration include collagen, hyaluronic acid, and fibrin.
Factors Inhibiting Stroma Regeneration
Inflammation, aging, and genetic mutations can impede stroma regeneration. These factors disrupt the delicate balance of cellular signaling pathways, leading to impaired tissue repair and fibrosis.
- Inflammation: Chronic inflammation can disrupt the normal regenerative process, leading to excessive scar formation and fibrosis. Inflammatory mediators, such as TNF-α and IL-1β, can induce fibroblast activation and collagen deposition, hindering tissue regeneration.
- Aging: As we age, our cells lose their regenerative capacity, leading to slower wound healing and increased fibrosis. The decline in growth factor production and the accumulation of senescent cells contribute to the age-related decline in stroma regeneration.
- Genetic Mutations: Genetic mutations can disrupt the signaling pathways involved in stroma regeneration, leading to impaired tissue repair and fibrosis. For example, mutations in genes involved in collagen synthesis or degradation can lead to abnormal scar formation and impaired wound healing.
Experimental Investigation of Stroma Regeneration
To investigate the impact of a specific factor on stroma regeneration, we can design an experiment using an animal model, such as a mouse. The experiment would involve two groups of mice: a control group and an experimental group.
- Control Group: This group would receive a sham treatment, such as a saline injection, to establish a baseline for stroma regeneration.
- Experimental Group: This group would receive the specific factor being investigated, such as a growth factor or a cytokine.
After a predetermined period, the mice would be euthanized, and their tissues would be analyzed to assess the extent of stroma regeneration. Histological analysis could be used to evaluate the amount of new tissue formation, while immunostaining could be used to identify the expression of specific markers associated with stroma regeneration.By comparing the results between the control and experimental groups, we can determine the impact of the specific factor on stroma regeneration.
This type of experiment can provide valuable insights into the mechanisms underlying stroma regeneration and help identify potential therapeutic targets for enhancing tissue repair.
Stroma Regeneration in Different Tissues: Does Stroma Regenerate

The regenerative capacity of stroma varies significantly across different tissues. Understanding these variations is crucial for developing targeted therapies for tissue repair and regeneration. This section explores the specific characteristics of stroma regeneration in various tissues, highlighting the unique challenges and opportunities associated with each.
Stroma Regeneration in Skin
The skin, our largest organ, has a remarkable capacity for regeneration. This is primarily due to the presence of a highly active population of stromal cells, including fibroblasts, keratinocytes, and endothelial cells. These cells contribute to the formation of new connective tissue, blood vessels, and the epidermis, respectively.
- Skin injuries, such as wounds and burns, trigger a complex cascade of events involving stromal cell proliferation, migration, and differentiation.
- Fibroblasts play a crucial role in wound healing, producing collagen and other extracellular matrix components that provide structural support and promote tissue repair.
- Keratinocytes, the primary cells of the epidermis, proliferate and migrate to close the wound, forming a new epithelial layer.
- Endothelial cells contribute to the formation of new blood vessels, ensuring adequate oxygen and nutrient supply to the healing wound.
However, skin regeneration can be compromised in certain conditions, such as chronic wounds, burns, and aging.
Stroma Regeneration in Liver
The liver is a remarkable organ known for its exceptional regenerative capacity. This ability is largely attributed to the presence of a specialized population of stromal cells called hepatic stellate cells (HSCs).
- HSCs are quiescent cells that reside in the space of Disse, a specialized compartment between hepatocytes and sinusoidal endothelial cells.
- Upon liver injury, HSCs undergo activation, transforming into myofibroblast-like cells that produce extracellular matrix components and contribute to tissue repair.
- Liver regeneration is a highly orchestrated process involving multiple cell types, including hepatocytes, cholangiocytes, and stromal cells.
- However, excessive scar formation, known as fibrosis, can impair liver function and lead to cirrhosis.
Understanding the mechanisms regulating HSC activation and fibrosis is crucial for developing therapies to promote liver regeneration and prevent disease progression.
Stroma Regeneration in Heart
The heart, unlike the skin and liver, has a limited capacity for regeneration. This is due to the relatively low number of resident stromal cells and the presence of a specialized extracellular matrix that limits cell migration and proliferation.
- Cardiac fibroblasts, the primary stromal cells in the heart, contribute to scar formation following myocardial infarction (heart attack). This scar tissue, while essential for structural integrity, can impair cardiac function.
- Recent research has focused on harnessing the potential of stem cells and biomaterials to enhance cardiac regeneration. These approaches aim to deliver stem cells to the injured heart, promoting the formation of new cardiomyocytes and blood vessels.
- Other strategies involve using biomaterials to create a scaffold that supports the growth of new heart tissue. This approach could potentially address the challenges associated with limited cell migration and proliferation in the heart.
Despite the challenges, ongoing research continues to explore novel approaches to stimulate cardiac regeneration and improve outcomes for patients with heart disease.
Table Summarizing Stroma Regeneration in Various Tissues
| Tissue | Key Stromal Cell Types | Regenerative Capacity | Challenges | Opportunities |
|---|---|---|---|---|
| Skin | Fibroblasts, keratinocytes, endothelial cells | High | Chronic wounds, burns, aging | Stem cell therapy, biomaterials, growth factors |
| Liver | Hepatic stellate cells (HSCs) | High | Fibrosis, cirrhosis | Targeting HSC activation, regenerative medicine |
| Heart | Cardiac fibroblasts | Low | Limited cell migration and proliferation, scar formation | Stem cell therapy, biomaterials, gene therapy |
Clinical Applications of Stroma Regeneration
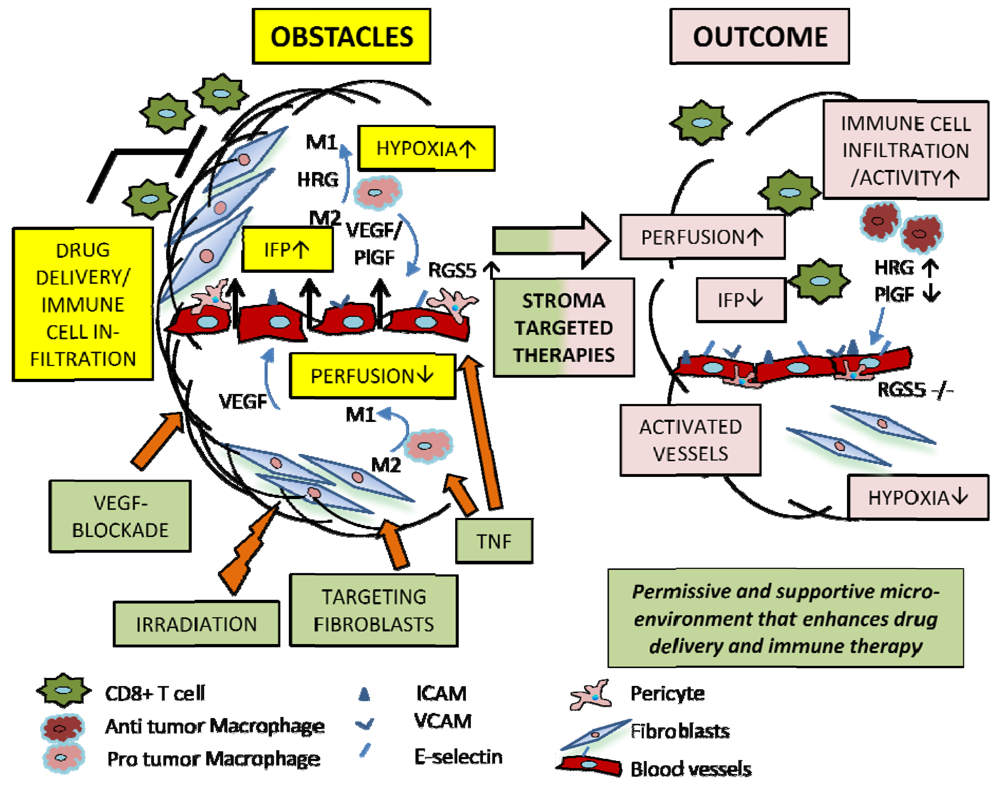
Stroma regeneration holds immense potential in the clinical realm, revolutionizing various medical practices and offering innovative solutions for treating a wide range of diseases and conditions. The ability to stimulate and control the regeneration of stromal tissues opens doors to advancements in wound healing, tissue engineering, and regenerative medicine.
Wound Healing
Stroma regeneration plays a pivotal role in wound healing, promoting the formation of new tissues and restoring the structural integrity of damaged areas. By stimulating the production of stromal cells, such as fibroblasts and endothelial cells, wound healing processes are accelerated, leading to faster closure and improved tissue regeneration.
“Stroma regeneration is essential for wound healing, promoting the formation of new tissues and restoring the structural integrity of damaged areas.”
- Enhanced Wound Closure: Stroma regeneration promotes the formation of granulation tissue, a vital component in wound closure. This tissue, rich in stromal cells, provides a scaffold for new tissue formation, facilitating faster wound closure and reducing the risk of complications.
- Improved Scar Formation: Stroma regeneration can influence the quality of scar formation, reducing the likelihood of hypertrophic or keloid scars. By promoting the production of collagen and other extracellular matrix components, stromal regeneration helps to create a more aesthetically pleasing and functional scar.
- Treatment of Chronic Wounds: Chronic wounds, such as diabetic ulcers and pressure sores, often fail to heal due to impaired stromal regeneration. By stimulating stromal cell proliferation and differentiation, therapeutic strategies aimed at promoting stroma regeneration can effectively treat these recalcitrant wounds.
Tissue Engineering
Stroma regeneration is a cornerstone of tissue engineering, enabling the creation of functional tissues and organs for transplantation. By harnessing the regenerative potential of stromal cells, scientists and engineers are developing novel approaches to address tissue and organ shortages.
- Biomaterial Scaffolds: Stroma regeneration can be integrated with biomaterial scaffolds to create three-dimensional structures that mimic the natural extracellular matrix of tissues. These scaffolds serve as templates for stromal cell growth and differentiation, leading to the formation of functional tissues.
- Cell-Based Therapies: Stroma regeneration can be harnessed to generate cell-based therapies for tissue repair and regeneration. By isolating and expanding stromal cells, such as mesenchymal stem cells, these cells can be used to regenerate damaged tissues or organs.
- Organ-on-a-Chip Technology: Stroma regeneration is playing a vital role in the development of organ-on-a-chip technology, which involves creating microfluidic devices that mimic the function of human organs. By incorporating stromal cells into these devices, researchers can study disease processes and test new drug candidates in a more physiologically relevant setting.
Regenerative Medicine
Stroma regeneration is at the forefront of regenerative medicine, offering hope for treating a wide range of diseases and conditions that were previously considered incurable. By stimulating the regeneration of damaged tissues and organs, regenerative medicine aims to restore function and improve the quality of life for patients.
- Cardiovascular Disease: Stroma regeneration is being explored for treating cardiovascular diseases, such as heart failure and myocardial infarction. By promoting the formation of new blood vessels and cardiomyocytes, stromal regeneration can improve heart function and reduce the risk of future cardiovascular events.
- Neurological Disorders: Stroma regeneration holds promise for treating neurological disorders, such as spinal cord injuries and stroke. By stimulating the regeneration of damaged neurons and glial cells, stromal regeneration can potentially restore neurological function and improve patient outcomes.
- Bone Regeneration: Stroma regeneration is being used to accelerate bone healing and regeneration in patients with fractures, bone defects, and osteoporosis. By promoting the formation of new bone tissue, stromal regeneration can lead to faster recovery and improved bone health.
Flowchart Illustrating Stroma Regeneration in Wound Healing
[Flowchart Description]The flowchart illustrates the steps involved in using stroma regeneration for wound healing. It starts with a wound injury, which triggers an inflammatory response. This response activates stromal cells, such as fibroblasts and endothelial cells, leading to the formation of granulation tissue. Granulation tissue provides a scaffold for new tissue formation, ultimately resulting in wound closure. Stroma regeneration also promotes the production of collagen and other extracellular matrix components, contributing to improved scar formation.
Future Directions in Stroma Regeneration Research
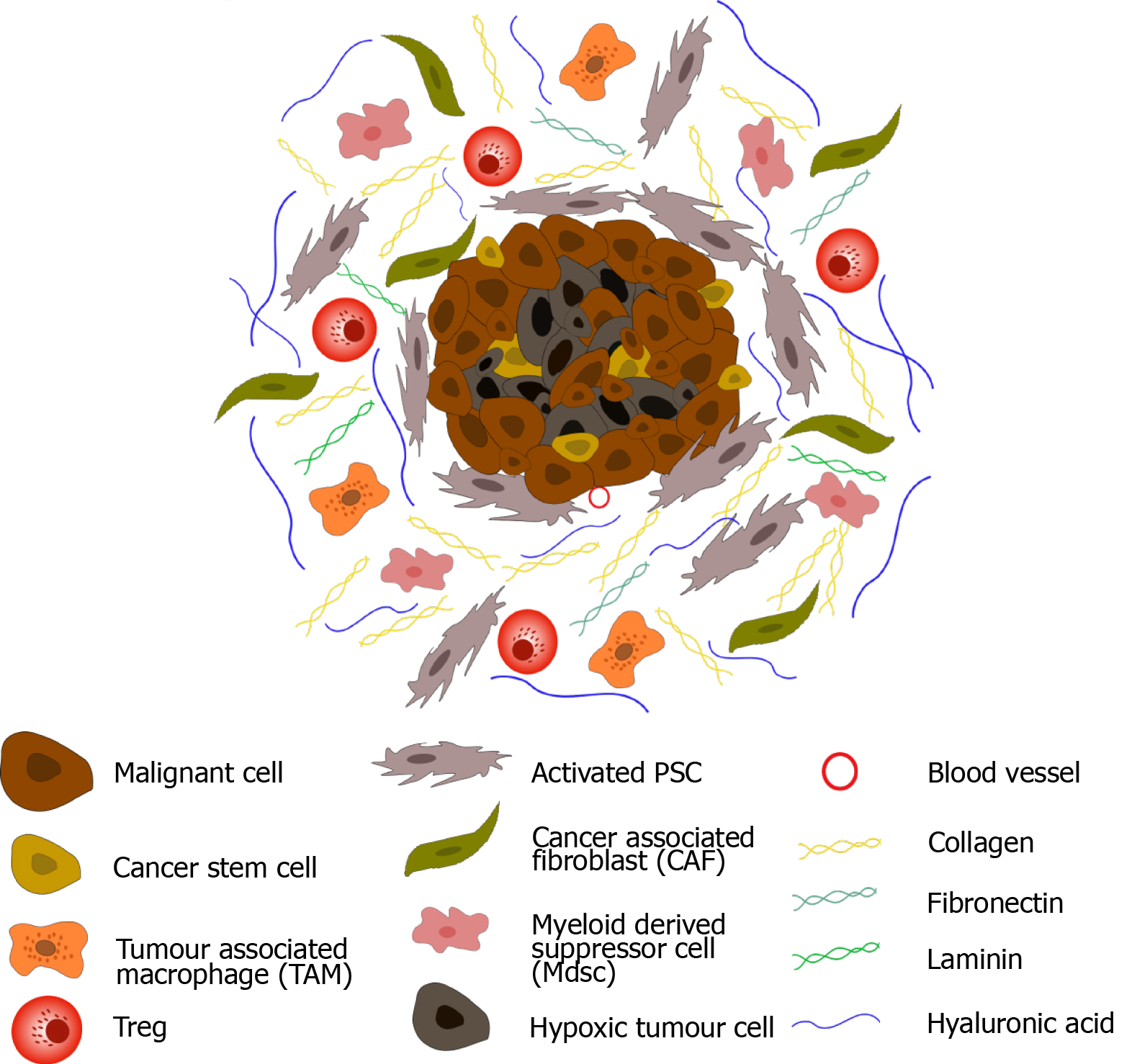
Stroma regeneration research is a rapidly evolving field with immense potential to revolutionize regenerative medicine. While significant progress has been made, several limitations and challenges still need to be addressed to fully harness the therapeutic potential of stroma regeneration. This section explores the current limitations and challenges, discusses promising future research directions, and examines the potential impact of emerging technologies on stroma regeneration research.
Addressing Current Limitations and Challenges, Does stroma regenerate
Understanding and manipulating stroma regeneration is a complex task, hindered by several limitations and challenges. One major challenge is the intricate and dynamic nature of the stromal microenvironment. The stroma is a complex network of cells and extracellular matrix components that constantly interact and influence each other. Deciphering the precise interplay of these factors and their contribution to regeneration is a significant hurdle.
Another limitation is the lack of reliable and reproducible methods for characterizing and quantifying stroma regeneration. Existing methods are often invasive, time-consuming, and limited in their ability to capture the full complexity of the stromal microenvironment. Furthermore, the clinical translation of stroma regeneration therapies is hampered by the lack of standardized protocols and robust preclinical models.
Promising Future Research Directions
Despite these challenges, several promising future research directions hold the key to advancing our understanding and application of stroma regeneration.
- Unveiling the Complexities of the Stromal Microenvironment: A deeper understanding of the intricate interplay between stromal cells and extracellular matrix components is crucial for developing effective regeneration strategies. This includes studying the role of different stromal cell types, their signaling pathways, and their interactions with the surrounding microenvironment. Advanced imaging techniques, single-cell analysis, and bioinformatics tools can be leveraged to decipher the complexities of the stromal microenvironment.
- Developing Novel Biomaterials and Scaffolds: Biomaterials and scaffolds play a critical role in supporting and guiding stroma regeneration. Future research should focus on developing biocompatible and bioresorbable materials that mimic the natural extracellular matrix and provide the appropriate cues for stromal cell recruitment, proliferation, and differentiation. Biomaterials incorporating growth factors, cytokines, and other bioactive molecules can be explored to enhance stromal regeneration.
- Exploring the Therapeutic Potential of Stem Cells: Stem cells, particularly mesenchymal stem cells (MSCs), hold immense potential for stroma regeneration. Research should focus on understanding the mechanisms by which MSCs contribute to stroma regeneration, optimizing their delivery methods, and exploring their use in combination with biomaterials and other therapeutic modalities.
- Harnessing the Power of Bioprinting: Bioprinting offers a promising approach to creating customized, three-dimensional tissues with precise control over cell placement and extracellular matrix composition. This technology can be used to engineer complex stromal structures for regenerative medicine applications.
Impact of Emerging Technologies
Emerging technologies are poised to revolutionize stroma regeneration research, accelerating progress and opening up new avenues for therapeutic development.
- Artificial Intelligence (AI): AI algorithms can analyze vast datasets from high-throughput screening experiments and clinical trials, identifying patterns and insights that could be missed by traditional methods. This can accelerate the discovery of novel therapeutic targets and strategies for stroma regeneration.
- Organ-on-a-Chip Technologies: Organ-on-a-chip models provide a more physiologically relevant platform for studying stroma regeneration in vitro. These models can be used to test the efficacy of new therapies, screen for drug candidates, and investigate the mechanisms of regeneration in a controlled environment.
- Gene Editing Technologies: CRISPR-Cas9 and other gene editing technologies offer the potential to modify stromal cells to enhance their regenerative capacity or target specific disease pathways. This could lead to personalized therapies for a wide range of diseases.
Understanding the intricate dance of cell proliferation, differentiation, and extracellular matrix remodeling that governs stroma regeneration is crucial for advancing tissue engineering and regenerative medicine. By harnessing the regenerative potential of stroma, we can pave the way for innovative therapies that restore tissue function and improve patient outcomes. The future of stroma regeneration research holds immense promise for addressing a wide range of medical challenges, from wound healing to organ transplantation.
Commonly Asked Questions
What are the main types of stromal cells?
Stromal cells are diverse and include fibroblasts, endothelial cells, pericytes, and immune cells. Each cell type contributes to the structural integrity and functional support of tissues.
How does inflammation affect stroma regeneration?
Inflammation can both promote and hinder stroma regeneration. While it is essential for clearing debris and initiating repair, excessive or chronic inflammation can disrupt the regenerative process and lead to scarring.
What are the potential applications of stroma regeneration in disease treatment?
Stroma regeneration holds promise for treating a wide range of diseases, including heart disease, liver disease, and diabetes. It can be used to promote tissue repair, regenerate damaged organs, and enhance the efficacy of other therapies.






