Does the stroma regenerate? This question delves into the fascinating world of tissue repair and renewal, where the intricate network of supporting structures within our organs plays a crucial role. The stroma, a complex tapestry of cells and extracellular matrix, provides essential scaffolding and signaling cues that govern the behavior of surrounding cells. Its ability to regenerate, therefore, holds immense implications for our understanding of tissue homeostasis, wound healing, and even the potential for regenerative medicine.
This exploration will unravel the secrets of stromal regeneration, delving into its intricate mechanisms, the factors that influence its capacity, and its relevance in various tissues and disease states. We will examine how the stroma responds to injury, the key players involved in its repair, and the potential for harnessing its regenerative power for therapeutic purposes. Join us on this journey to discover the remarkable resilience of the stroma and its vital role in maintaining our bodily integrity.
Stroma
The stroma is a supportive framework found within various tissues and organs, providing structural integrity and contributing to their overall function. It acts as a scaffold for cells, facilitating communication and interaction between them, and ensuring the proper organization of the tissue.
Stroma Composition
The stroma is a complex structure composed of cellular and extracellular components.
- Cellular Components: Stromal cells are diverse and play crucial roles in tissue maintenance and function. Some common examples include:
- Fibroblasts: These cells are responsible for producing and maintaining the extracellular matrix, providing structural support and tensile strength to tissues. They synthesize collagen, elastin, and other proteins that form the fibrous network of the stroma.
- Smooth Muscle Cells: Found in the stroma of certain organs, such as the blood vessels, they contribute to vascular tone and regulation of blood flow.
- Endothelial Cells: These cells line the blood vessels and lymphatic vessels, facilitating the exchange of nutrients and waste products between the blood and tissues.
- Immune Cells: The stroma also contains immune cells, such as macrophages, lymphocytes, and mast cells, which play a vital role in the immune response and defense against pathogens.
- Extracellular Components: The extracellular matrix (ECM) is a complex network of proteins and polysaccharides that provides structural support, regulates cell behavior, and facilitates communication between cells.
- Collagen: A major structural protein, collagen fibers provide tensile strength and resistance to stretching.
- Elastin: Elastin fibers provide elasticity and allow tissues to return to their original shape after stretching or compression.
- Glycosaminoglycans (GAGs): These polysaccharides, such as hyaluronic acid, chondroitin sulfate, and heparin sulfate, contribute to the hydration and viscoelastic properties of the stroma.
- Proteoglycans: These molecules consist of a core protein attached to GAGs. They play a role in cell adhesion, migration, and signaling.
Stroma in Different Tissues and Organs
The stroma varies in composition and function depending on the specific tissue or organ.
- Connective Tissues: The stroma is the primary component of connective tissues, providing structural support and connecting different tissues.
- Muscles: The stroma in muscles provides a framework for muscle fibers, allowing for efficient contraction and movement.
- Blood Vessels: The stroma of blood vessels includes smooth muscle cells and endothelial cells, regulating blood flow and nutrient exchange.
- Organs: The stroma of organs, such as the liver, kidney, and pancreas, supports the functional parenchyma and facilitates the exchange of substances between the parenchyma and the blood.
Regeneration
Regeneration, in the context of tissue repair and renewal, is the process by which damaged or lost tissues are replaced with new, functional tissues. It’s a remarkable biological process that allows organisms to recover from injuries, maintain tissue homeostasis, and even regenerate entire organs in some cases.
Mechanisms of Regeneration
The process of regeneration involves a complex interplay of various cellular and molecular mechanisms. These mechanisms can be broadly categorized into three main phases: cell division, differentiation, and remodeling.
- Cell Division: This is the fundamental process that underlies regeneration. Damaged tissues trigger the proliferation of stem cells and progenitor cells, which are specialized cells capable of dividing and giving rise to new cells. These newly formed cells then replace the lost or damaged cells, contributing to the restoration of tissue structure and function.
- Differentiation: Once new cells are generated through cell division, they undergo differentiation, a process where they acquire specific characteristics and functions appropriate for the tissue they are replacing. This involves the activation of specific genes and the production of proteins that determine the cell’s identity and role.
- Remodeling: After new cells have been generated and differentiated, the tissue undergoes remodeling, a process that involves the reorganization and maturation of the newly formed tissue. This phase ensures that the regenerated tissue is properly integrated into the surrounding tissue and that its structure and function are restored.
Regeneration vs. Other Forms of Tissue Repair
Regeneration is not the only way that tissues can repair themselves. Other forms of tissue repair, such as fibrosis, also play a role in restoring tissue integrity. However, these processes differ significantly from regeneration in terms of their outcomes and the mechanisms involved.
- Fibrosis: Fibrosis is a process where damaged tissues are replaced by scar tissue, which is composed primarily of collagen. While fibrosis can help to stabilize the injured area and prevent further damage, it does not restore the original tissue function. Unlike regeneration, which involves the formation of new functional tissue, fibrosis results in the formation of non-functional scar tissue, which can impair tissue function and limit movement.
Factors Influencing Stroma Regeneration
The regenerative capacity of the stroma, the supporting framework of tissues and organs, is influenced by a complex interplay of intrinsic and extrinsic factors. Understanding these factors is crucial for developing strategies to promote stromal regeneration and repair in various disease states.
Growth Factors and Cytokines
Growth factors and cytokines play pivotal roles in regulating stromal regeneration. These signaling molecules, secreted by various cell types, act as molecular messengers, stimulating cell proliferation, differentiation, and migration, all essential for tissue repair.
- Fibroblast Growth Factors (FGFs): FGFs, a family of signaling proteins, are key regulators of stromal cell proliferation and differentiation. They stimulate the formation of new blood vessels (angiogenesis) and promote the production of extracellular matrix components, crucial for tissue structure and integrity. For example, FGF-2, also known as basic fibroblast growth factor (bFGF), is a potent mitogen for fibroblasts and other stromal cells, promoting their proliferation and differentiation.
- Transforming Growth Factor-beta (TGF-β): TGF-β is a pleiotropic cytokine involved in a wide range of cellular processes, including cell growth, differentiation, and extracellular matrix production. It plays a crucial role in wound healing and tissue repair, promoting fibroblast migration and collagen deposition. However, excessive TGF-β signaling can contribute to fibrosis, a condition characterized by excessive scar tissue formation.
- Platelet-Derived Growth Factor (PDGF): PDGF is released from platelets during wound healing and plays a significant role in stromal cell proliferation and migration. It stimulates the recruitment of fibroblasts and other stromal cells to the injury site, promoting tissue repair.
- Cytokines: Cytokines, such as interleukin-1 (IL-1) and tumor necrosis factor-alpha (TNF-α), are involved in inflammation and wound healing. They can stimulate the production of growth factors and chemokines, attracting immune cells and promoting stromal regeneration.
Signaling Pathways
The actions of growth factors and cytokines are mediated through specific signaling pathways, which regulate cellular responses and ultimately influence stromal regeneration.
- MAPK Pathway: The mitogen-activated protein kinase (MAPK) pathway is a crucial signaling pathway involved in cell proliferation, differentiation, and survival. Growth factors like FGFs and PDGF activate the MAPK pathway, leading to downstream signaling events that promote stromal cell growth and regeneration.
- PI3K/AKT Pathway: The phosphatidylinositol 3-kinase (PI3K)/AKT pathway is another important signaling pathway involved in cell survival, proliferation, and angiogenesis. Growth factors like IGF-1 and PDGF activate the PI3K/AKT pathway, promoting stromal cell survival and contributing to tissue regeneration.
- Wnt Pathway: The Wnt pathway is a complex signaling pathway involved in cell proliferation, differentiation, and tissue homeostasis. It plays a role in stromal regeneration by regulating the proliferation and differentiation of stromal cells, contributing to tissue repair and regeneration.
Age, Disease, and Injury
Stromal regeneration is influenced by factors such as age, disease, and injury.
- Age: The regenerative capacity of the stroma declines with age. This is due to several factors, including reduced growth factor production, decreased cell proliferation, and impaired extracellular matrix remodeling. As a result, older individuals often experience slower wound healing and reduced tissue regeneration.
- Disease: Certain diseases, such as diabetes and chronic inflammation, can impair stromal regeneration. These conditions can disrupt signaling pathways, alter growth factor expression, and promote fibrosis, hindering tissue repair and regeneration.
- Injury: The severity and nature of injury can significantly impact stromal regeneration. Severe injuries, such as burns or large wounds, often require more extensive tissue repair and may lead to scar formation. Chronic injuries, such as those associated with osteoarthritis, can lead to progressive degeneration of the stroma and impaired regeneration.
Stroma Regeneration in Specific Tissues
The regenerative capacity of stroma varies significantly across different tissues. Some tissues, like the liver and skin, exhibit remarkable regenerative abilities, while others have limited regenerative potential. This section delves into the specific mechanisms of stromal regeneration in tissues with high regenerative capacity, highlighting the differences in their regenerative processes.
Liver Regeneration
The liver possesses an exceptional capacity for regeneration. After partial hepatectomy (surgical removal of a portion of the liver), the remaining liver tissue undergoes a remarkable process of regeneration, restoring its original size and function. This process involves a complex interplay of signaling pathways, growth factors, and cellular proliferation.
- Growth factors: Hepatocyte growth factor (HGF) and transforming growth factor-alpha (TGF-α) play crucial roles in stimulating hepatocyte proliferation. These growth factors bind to their respective receptors on hepatocytes, triggering downstream signaling cascades that promote cell cycle progression and DNA synthesis.
- Cytokines: Cytokines, such as interleukin-6 (IL-6) and tumor necrosis factor-alpha (TNF-α), are involved in the inflammatory response that accompanies liver injury. While initially contributing to inflammation, they also play a role in activating hepatocyte proliferation and regeneration.
- Extracellular matrix remodeling: The extracellular matrix (ECM) surrounding hepatocytes undergoes significant remodeling during regeneration. Degradation of the ECM by matrix metalloproteinases (MMPs) allows for cell migration and proliferation, while the deposition of new ECM components provides structural support for the regenerating tissue.
Skin Regeneration
The skin is another tissue with a high regenerative capacity. The epidermis, the outermost layer of the skin, undergoes constant renewal, with new cells constantly being generated from the basal layer and migrating to the surface. This process is essential for maintaining the integrity of the skin barrier and protecting the body from external insults.
- Stem cells: The basal layer of the epidermis contains stem cells that are responsible for generating new epidermal cells. These stem cells divide asymmetrically, producing one daughter cell that remains a stem cell and another daughter cell that differentiates into a specific cell type, such as a keratinocyte.
- Growth factors: Epidermal growth factor (EGF) and keratinocyte growth factor (KGF) play important roles in stimulating keratinocyte proliferation and differentiation. These growth factors bind to their respective receptors on keratinocytes, activating signaling pathways that promote cell cycle progression and gene expression.
- Extracellular matrix remodeling: The ECM of the dermis, the layer beneath the epidermis, also undergoes remodeling during skin regeneration. MMPs degrade the ECM, allowing for cell migration and proliferation, while new ECM components are deposited to provide structural support for the regenerating tissue.
Comparison of Regenerative Capacity, Does the stroma regenerate
While the liver and skin exhibit remarkable regenerative abilities, other tissues have limited regenerative potential. For example, the heart has a very limited capacity for regeneration, and damage to the heart muscle often leads to permanent scarring. This difference in regenerative capacity is attributed to several factors, including:
- Cell type: Tissues with high regenerative capacity, like the liver and skin, are composed of cells that have a high proliferative potential. In contrast, tissues with limited regenerative capacity, like the heart, are composed of cells that have a limited proliferative potential.
- ECM composition: The ECM of different tissues varies in its composition and structure. The ECM of tissues with high regenerative capacity is often more dynamic and responsive to injury, allowing for cell migration and proliferation. In contrast, the ECM of tissues with limited regenerative capacity is often more rigid and less responsive to injury, hindering regeneration.
- Signaling pathways: The signaling pathways involved in regeneration differ between tissues. Tissues with high regenerative capacity often have more robust and efficient signaling pathways that promote cell proliferation and differentiation. In contrast, tissues with limited regenerative capacity may have less efficient or even impaired signaling pathways, limiting regeneration.
Stroma Regeneration and Disease: Does The Stroma Regenerate

The intricate dance of stromal regeneration plays a crucial role in maintaining tissue homeostasis and orchestrating responses to injury and disease. Understanding the mechanisms governing stromal regeneration is vital for developing therapeutic strategies that promote healing and combat disease.
Stroma Regeneration in Wound Healing and Tissue Repair
Stroma regeneration is a fundamental process in wound healing, ensuring the restoration of damaged tissues. Following injury, stromal cells, including fibroblasts and mesenchymal stem cells, are activated, proliferate, and migrate to the wound site. These cells secrete extracellular matrix components, such as collagen, elastin, and proteoglycans, providing structural support and guiding tissue repair. The intricate interplay between stromal cells and other cell types, such as epithelial cells and immune cells, contributes to the coordinated process of wound closure and tissue regeneration.
Potential of Stroma Regeneration in Regenerative Medicine and Tissue Engineering
The remarkable regenerative capacity of stromal cells has sparked significant interest in regenerative medicine and tissue engineering. Scientists are exploring the potential of stromal cells to generate new tissues and organs, offering hope for treating various diseases and injuries. For example, stromal cells can be used to create bioengineered tissues for skin grafts, cartilage repair, and bone regeneration. The ability to manipulate stromal cell behavior and promote their differentiation into specific cell types holds immense promise for developing novel therapies for a wide range of conditions.
Diseases Affecting Stroma Regeneration
While stromal regeneration is essential for tissue repair, certain diseases can disrupt this process, leading to pathological consequences.
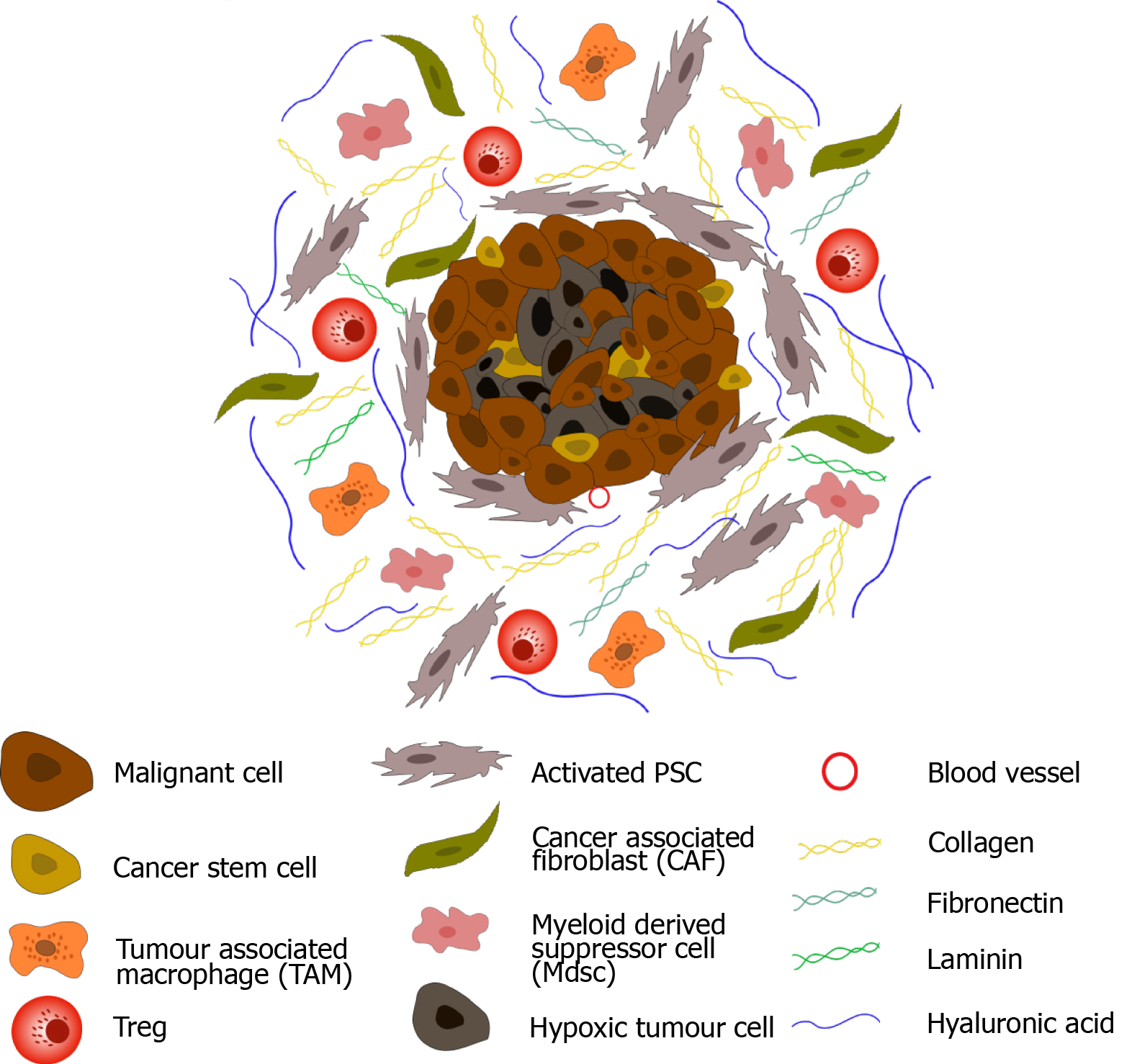
Cancer
Cancer cells often exploit stromal regeneration pathways to fuel their growth and spread. For instance, tumor cells can secrete factors that promote stromal cell proliferation and angiogenesis, providing a favorable environment for tumor growth. The interplay between cancer cells and the stroma is complex and multifaceted, influencing tumor progression, metastasis, and response to therapy.
Fibrosis
Fibrosis, characterized by excessive deposition of extracellular matrix components, is a common consequence of chronic inflammation and tissue injury. In fibrosis, stromal cells undergo aberrant activation and differentiation, leading to the accumulation of scar tissue that can impair organ function. Understanding the mechanisms underlying fibrotic scarring and developing strategies to modulate stromal cell behavior are crucial for treating fibrotic diseases.
Future Directions in Stroma Regeneration Research
The field of stromal regeneration is rapidly evolving, driven by advancements in our understanding of stromal biology and the development of novel technologies. This research area holds immense potential for treating a wide range of diseases and injuries, offering hope for restoring tissue function and improving patient outcomes.
Potential Therapeutic Strategies
Several promising therapeutic strategies are emerging to enhance stromal regeneration. These strategies aim to manipulate the complex interplay of cellular and molecular factors that govern stromal repair and remodeling.
- Cell-based therapies: Harnessing the regenerative potential of stromal cells, such as mesenchymal stem cells (MSCs) and fibroblasts, is a key focus. MSCs, with their ability to differentiate into various cell types and secrete growth factors, are being investigated for their therapeutic potential in tissue regeneration. For instance, MSCs derived from bone marrow or adipose tissue have shown promising results in clinical trials for treating conditions like osteoarthritis and heart failure.
- Biomaterials and scaffolds: Biocompatible materials, often designed to mimic the extracellular matrix (ECM), are being developed to provide structural support and guide the regeneration process. These scaffolds can act as a framework for cell attachment, migration, and proliferation, facilitating the formation of new stromal tissue.
- Growth factors and cytokines: Specific growth factors and cytokines, such as platelet-derived growth factor (PDGF) and transforming growth factor-beta (TGF-β), play critical roles in stimulating stromal cell proliferation, differentiation, and ECM production. These factors are being investigated for their potential to enhance stromal regeneration, either alone or in combination with other therapies.
- Gene therapy: Gene therapy approaches aim to deliver therapeutic genes that can enhance stromal regeneration by promoting cell survival, proliferation, or the production of specific growth factors. For example, gene therapy strategies targeting the expression of genes involved in ECM synthesis or angiogenesis are being explored for their potential to improve stromal regeneration.
Ethical Considerations and Challenges
The manipulation of stromal regeneration, while promising, raises important ethical considerations and challenges.
- Safety and efficacy: Ensuring the safety and efficacy of stromal regeneration therapies is paramount. Rigorous preclinical and clinical studies are crucial to assess potential risks and benefits. Furthermore, long-term follow-up is essential to monitor the long-term effects of these therapies.
- Accessibility and cost: Ensuring that stromal regeneration therapies are accessible to all patients, regardless of their socioeconomic status, is a critical concern. The cost of developing and delivering these therapies can be substantial, raising questions about equitable access.
- Off-target effects: Manipulating stromal regeneration could potentially have unintended consequences on other tissues or organs. Therefore, careful consideration of potential off-target effects is crucial to ensure the safety and efficacy of these therapies.
- Ethical implications of using stem cells: The use of stem cells in stromal regeneration therapies raises ethical concerns, particularly regarding the source of these cells and their potential for uncontrolled proliferation. Ethical guidelines and regulatory frameworks are essential to ensure responsible use of stem cell technologies.
The ability of the stroma to regenerate is a testament to the remarkable plasticity and resilience of our tissues. Understanding the intricate mechanisms of stromal regeneration opens up exciting avenues for therapeutic interventions, particularly in the realm of regenerative medicine and tissue engineering. As we delve deeper into the complexities of stromal regeneration, we uncover not only its crucial role in maintaining tissue homeostasis but also its potential to revolutionize the way we approach tissue repair and disease treatment.
The future of stromal regeneration research holds immense promise for enhancing our understanding of tissue biology and ultimately improving human health.
Quick FAQs
What are some examples of tissues with high regenerative capacity?
The liver and skin are known for their exceptional regenerative abilities. The liver possesses remarkable regenerative potential, able to regenerate large portions of lost tissue. Similarly, the skin’s ability to repair wounds and regenerate lost layers is a testament to its remarkable regenerative capacity.
How does age affect stromal regeneration?
As we age, the regenerative capacity of our tissues, including the stroma, generally declines. This decline is attributed to various factors, including changes in cellular signaling pathways, reduced cell division, and alterations in the extracellular matrix. As a result, wound healing and tissue repair may become slower and less efficient in older individuals.
Can stromal regeneration be manipulated for therapeutic purposes?
Yes, manipulating stromal regeneration for therapeutic purposes is an active area of research. Scientists are exploring various strategies, including the use of growth factors, stem cells, and biomaterials, to enhance stromal regeneration and promote tissue repair in various disease states.






