How can stroma cells cause hyperplasia? This question delves into the intricate relationship between these supporting cells and the growth of tissues. Stroma cells, often considered the “backbone” of tissues, play a vital role in maintaining tissue structure and function. Hyperplasia, on the other hand, refers to an increase in the number of cells in a tissue, often driven by specific signaling pathways.
Understanding how stroma cells contribute to hyperplasia is crucial for comprehending the development of various diseases and exploring potential therapeutic interventions.
Stroma cells, found in various tissues throughout the body, interact with neighboring cells, providing structural support and regulating their behavior. Hyperplasia, a common phenomenon in both physiological and pathological processes, involves an increase in cell number within a tissue, driven by factors that promote cell division. The interplay between stroma cells and target cells in tissues can lead to hyperplasia, a process that can be either beneficial or detrimental depending on the context.
Introduction to Stroma Cells and Hyperplasia
The intricate tapestry of life is woven from countless threads, each playing a crucial role in maintaining the delicate balance of our existence. Among these threads are the humble stroma cells, often overshadowed by the more glamorous epithelial cells, yet silently orchestrating the very foundation of our tissues. Stroma cells, the unsung heroes of our biological symphony, provide the structural support and microenvironment that allows epithelial cells to flourish and perform their specialized functions.
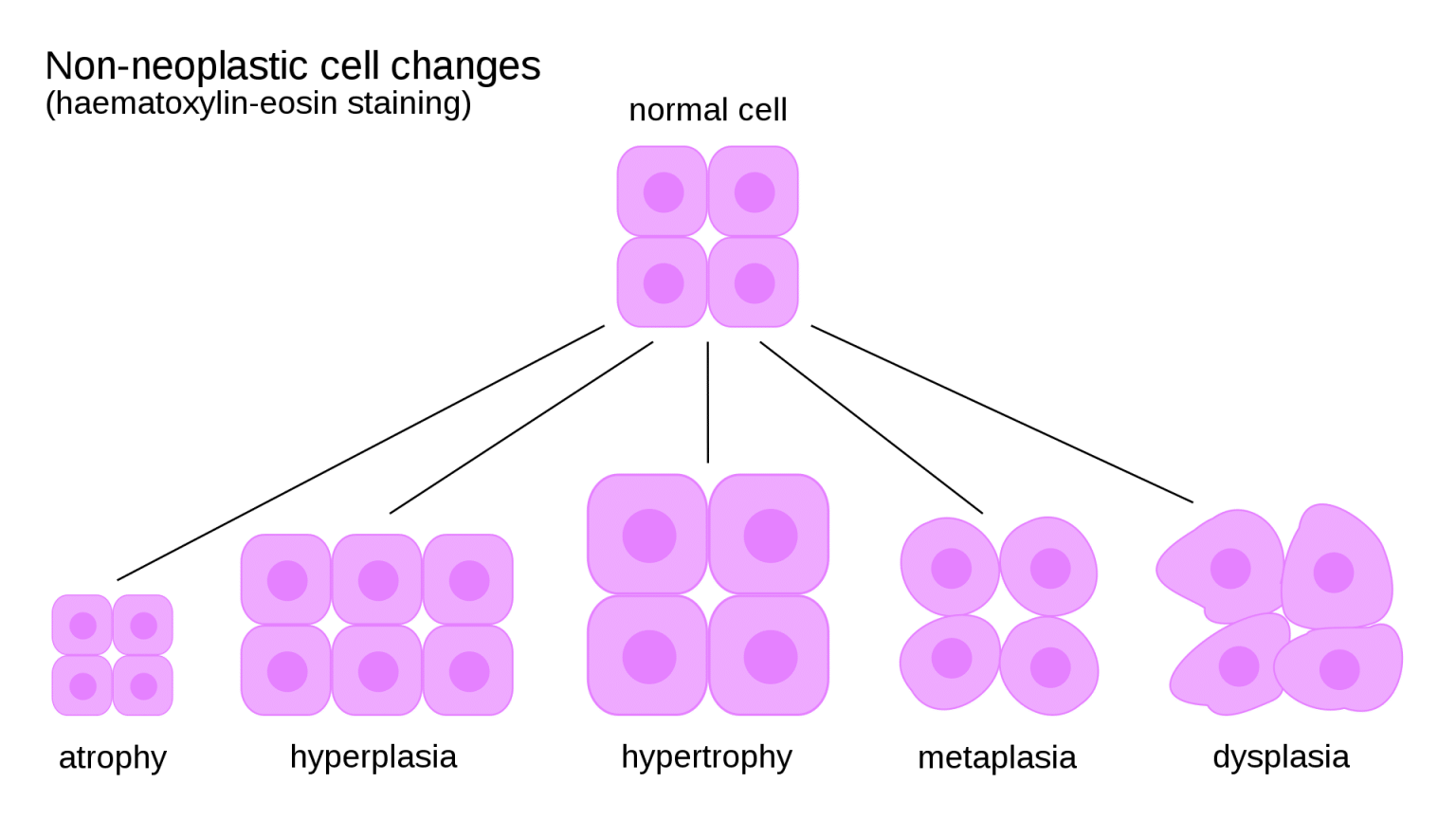
Hyperplasia, a process of controlled cellular proliferation, is a natural response to physiological demands or environmental cues, and it is often influenced by the interplay between stroma cells and their epithelial counterparts.Hyperplasia, a symphony of cellular growth, is a dynamic process that involves an increase in the number of cells within a tissue or organ. This controlled proliferation is not simply a random event but a carefully orchestrated response to a variety of signals.
The mechanisms driving hyperplasia are intricate, involving a delicate dance between growth factors, hormones, and intracellular signaling pathways. Hyperplasia can be classified into two primary types: physiological and pathological. Physiological hyperplasia is a normal, adaptive response to physiological demands, such as the growth of the uterus during pregnancy or the thickening of the skin in response to repeated friction.
Pathological hyperplasia, on the other hand, is an abnormal increase in cell number that can be a precursor to cancer or other diseases.
Stroma Cells in Hyperplasia
Stroma cells, often the unsung heroes of tissue architecture, play a multifaceted role in the regulation of hyperplasia. They provide the structural support, secrete essential growth factors, and create a microenvironment that influences the behavior of epithelial cells. In the context of hyperplasia, stroma cells can act as both promoters and inhibitors of cell proliferation. They can release growth factors that stimulate epithelial cell division, but they can also produce factors that suppress growth or induce apoptosis.
The precise role of stroma cells in hyperplasia is highly context-dependent, varying depending on the specific tissue, the type of hyperplasia, and the underlying physiological or pathological stimuli.
Examples of Stroma Cell Contribution to Hyperplasia
The interplay between stroma cells and epithelial cells in hyperplasia is a complex dance that unfolds in a variety of tissues. In the context of prostate hyperplasia, stromal cells release factors like fibroblast growth factor (FGF) and insulin-like growth factor (IGF), which stimulate epithelial cell proliferation and contribute to the enlargement of the prostate gland. In the breast, stromal cells can play a role in the development of ductal hyperplasia, a condition characterized by an increase in the number of epithelial cells lining the breast ducts.
These examples highlight the diverse and crucial role that stroma cells play in the regulation of hyperplasia, a process that is essential for tissue development, regeneration, and response to environmental cues.
Mechanisms of Stroma Cell-Mediated Hyperplasia
The intricate dance between stroma cells and target cells is a symphony of signals, orchestrating growth and development. Stroma cells, like the backstage crew, play a crucial role in influencing the proliferation of target cells, acting as the silent directors of hyperplasia. This intricate interplay involves a complex network of signaling pathways, with growth factors and cytokines acting as the messengers.
Growth Factors and Cytokines
Growth factors and cytokines, secreted by stroma cells, act as potent signals that can trigger the proliferation of target cells. These molecular messengers bind to specific receptors on the target cell surface, initiating a cascade of intracellular events.
Growth factors and cytokines act as molecular messengers, binding to specific receptors on the target cell surface, triggering a cascade of intracellular events.
- Growth factors, like fibroblast growth factor (FGF) and epidermal growth factor (EGF), promote cell division and survival. These factors stimulate the expression of genes involved in cell cycle progression, ultimately driving the target cells to replicate.
- Cytokines, like interleukin-6 (IL-6) and tumor necrosis factor-alpha (TNF-α), can also influence proliferation. While some cytokines, like IL-6, promote cell growth, others, like TNF-α, can have both stimulatory and inhibitory effects, depending on the context.
Examples of Stroma Cell-Derived Factors
Several specific factors secreted by stroma cells are known to promote hyperplasia in various tissues.
- Fibroblast growth factor (FGF): FGFs are a family of growth factors that play a crucial role in wound healing and tissue regeneration. They stimulate the proliferation of a variety of cell types, including epithelial cells, fibroblasts, and endothelial cells. In the context of prostate hyperplasia, FGFs have been implicated in the increased growth of prostate epithelial cells.
- Hepatocyte growth factor (HGF): HGF is a potent mitogen for epithelial cells, promoting their proliferation and survival. It has been shown to contribute to the development of liver hyperplasia in response to injury or inflammation.
- Insulin-like growth factor (IGF): IGFs are a family of hormones that promote cell growth and development. They have been implicated in the development of hyperplasia in various tissues, including the prostate, breast, and colon.
Stroma Cells in Different Hyperplastic Conditions
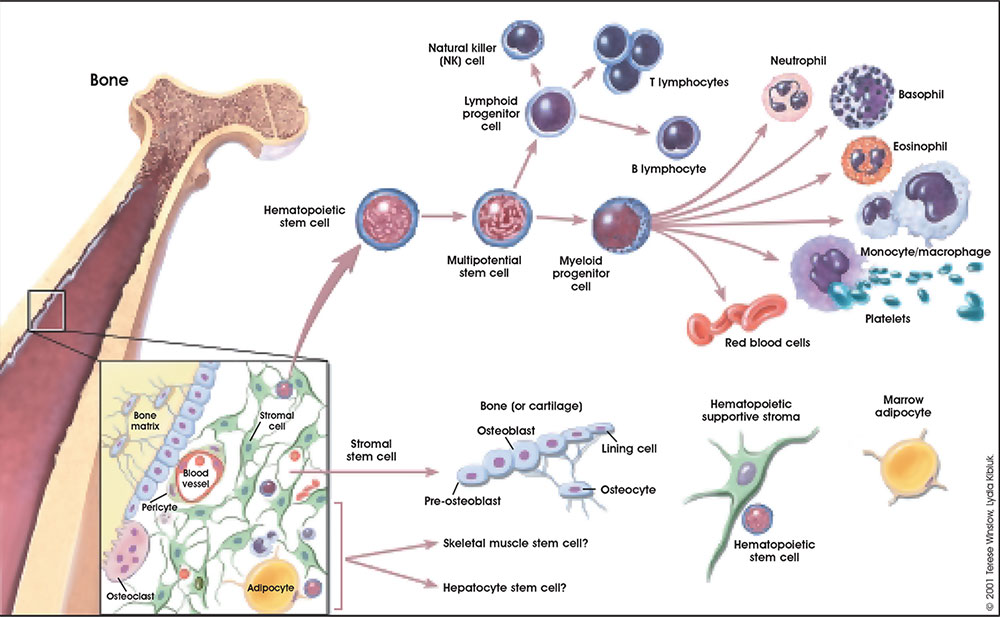
Stroma cells play a multifaceted role in various hyperplastic conditions, acting as both contributors and regulators of excessive tissue growth. Understanding the specific interplay between stroma cells and other cell types within these conditions is crucial for developing targeted therapeutic strategies.
Stroma Cells in Prostate Hyperplasia
Prostate hyperplasia, a common condition in aging men, is characterized by an increase in the size of the prostate gland, leading to urinary difficulties. Stroma cells play a crucial role in the development of this condition, primarily through their interaction with epithelial cells. In prostate hyperplasia, stromal cells undergo an increase in proliferation, leading to an expansion of the stromal compartment.
This stromal expansion, in turn, compresses the surrounding epithelial cells, causing them to proliferate and contribute to the overall enlargement of the gland.
Stroma Cells in Endometrial Hyperplasia
Endometrial hyperplasia, a condition marked by an abnormal thickening of the uterine lining, is often associated with an increased risk of endometrial cancer. Stroma cells contribute to this condition by providing a supportive environment for the proliferation of endometrial epithelial cells. They secrete growth factors, such as insulin-like growth factor (IGF) and transforming growth factor-beta (TGF-β), which promote the growth and proliferation of epithelial cells.
In addition, stromal cells can influence the expression of estrogen receptors in epithelial cells, making them more sensitive to the proliferative effects of estrogen.
Stroma Cells in Fibroid Development
Uterine fibroids, benign tumors of the uterus, are another example of a condition where stroma cells play a significant role. Fibroids are primarily composed of smooth muscle cells, but their growth is heavily influenced by the surrounding stroma. Stroma cells in fibroids secrete various growth factors, including platelet-derived growth factor (PDGF) and fibroblast growth factor (FGF), which stimulate the proliferation and differentiation of smooth muscle cells, contributing to fibroid growth.
Stroma Cells in Other Hyperplastic Conditions
Stroma cells are implicated in a wide range of hyperplastic conditions, including:
- Breast Hyperplasia: Stroma cells in the breast contribute to hyperplasia by secreting growth factors that promote the proliferation of epithelial cells.
- Liver Hyperplasia: Stroma cells in the liver play a role in regenerative hyperplasia following injury, secreting factors that promote the proliferation of hepatocytes.
- Skin Hyperplasia: Stroma cells in the skin contribute to hyperplastic conditions such as psoriasis and keratosis, by secreting factors that promote the proliferation of keratinocytes.
“The intricate interplay between stroma cells and epithelial cells is essential for maintaining tissue homeostasis. However, disruptions in this delicate balance can lead to hyperplastic conditions, highlighting the importance of understanding the mechanisms underlying stroma cell-mediated hyperplasia.”
Potential Therapeutic Implications
The intricate dance between stroma cells and hyperplasia presents a unique opportunity to modulate tissue growth and potentially alleviate disease states. By targeting stroma cell activity, we can explore avenues for both inhibiting and enhancing hyperplasia, paving the way for novel therapeutic approaches.
Strategies for Modulating Stroma Cell-Mediated Hyperplasia
Understanding the mechanisms by which stroma cells influence hyperplasia allows us to develop targeted strategies to manipulate their activity.
- Inhibition of Stroma Cell-Mediated Hyperplasia:
- Targeting Growth Factors: Blocking the production or signaling of growth factors like FGF, VEGF, and TGF-β, which are often secreted by stroma cells, can effectively inhibit hyperplasia. Examples include monoclonal antibodies against VEGF (bevacizumab) or inhibitors of FGF receptors (FGFR inhibitors). These agents are already used in cancer therapy and may be repurposed for other hyperplastic conditions.
- Targeting Stroma Cell Signaling Pathways: Inhibiting key signaling pathways within stroma cells, such as the PI3K/AKT/mTOR pathway, can disrupt their ability to promote proliferation and survival of target cells. This approach has shown promise in preclinical studies and is being investigated for various hyperplastic diseases.
- Stroma Cell Depletion: Strategies like antibody-dependent cellular cytotoxicity (ADCC) or cytotoxic chemotherapy can be used to selectively eliminate stroma cells, reducing their contribution to hyperplasia. However, this approach may have off-target effects and needs careful consideration.
- Enhancement of Stroma Cell-Mediated Hyperplasia:
- Stroma Cell-Based Therapy: Engineering stroma cells to express growth factors or other therapeutic molecules could be used to promote tissue regeneration and repair in conditions like wound healing or tissue loss. This approach has shown potential in preclinical studies and is being explored for various regenerative medicine applications.
- Modulation of Stroma Cell Microenvironment: Altering the microenvironment surrounding stroma cells, such as by delivering oxygen or specific growth factors, can influence their activity and promote tissue regeneration. This approach is being explored for wound healing and tissue engineering applications.
Therapeutic Approaches and Mechanisms of Action
| Approach | Mechanism of Action | Example | Potential Applications |
|---|---|---|---|
| Growth Factor Inhibition | Blocking the production or signaling of growth factors, such as VEGF or FGF, secreted by stroma cells | Bevacizumab (anti-VEGF antibody) | Cancer therapy, diabetic retinopathy, wet age-related macular degeneration |
| Stroma Cell Signaling Pathway Inhibition | Inhibiting key signaling pathways within stroma cells, such as the PI3K/AKT/mTOR pathway | PI3K inhibitors, mTOR inhibitors | Cancer therapy, fibrosis, inflammatory diseases |
| Stroma Cell Depletion | Targeting stroma cells for elimination using antibody-dependent cellular cytotoxicity (ADCC) or cytotoxic chemotherapy | Rituximab (anti-CD20 antibody) | Cancer therapy, autoimmune diseases |
| Stroma Cell-Based Therapy | Engineering stroma cells to express growth factors or other therapeutic molecules for tissue regeneration | Stroma cell-based therapy for wound healing, tissue engineering | Wound healing, tissue engineering, regenerative medicine |
| Modulation of Stroma Cell Microenvironment | Altering the microenvironment surrounding stroma cells to influence their activity | Oxygen therapy, growth factor delivery | Wound healing, tissue engineering, regenerative medicine |
Research Directions and Future Perspectives: How Can Stroma Cells Cause Hyperplasia
The intricate dance between stroma cells and hyperplasia presents a fascinating and complex landscape ripe for exploration. Unraveling the mysteries of this interaction holds the key to unlocking novel therapeutic strategies for a wide range of diseases.
The Role of Stroma Cells in Different Hyperplastic Conditions
A deeper understanding of the diverse roles of stroma cells in various hyperplastic conditions is paramount. The mechanisms by which stroma cells contribute to hyperplasia in different tissues and organs remain largely unexplored. This knowledge gap hinders our ability to develop targeted therapies that effectively address the underlying causes of hyperplasia.
Identifying Stroma Cell Subtypes and Their Specific Functions
The heterogeneity of stroma cells within different tissues necessitates a detailed investigation into the specific subtypes and their unique roles in hyperplasia. Identifying and characterizing these subtypes, along with their molecular signatures, will pave the way for developing targeted therapies that selectively modulate the activity of specific stroma cell populations.
The Impact of Stroma Cell-Derived Factors on Hyperplasia
Stroma cells are known to secrete a plethora of factors that can influence the behavior of surrounding cells, including those involved in hyperplasia. A comprehensive analysis of the molecular repertoire of these factors, their specific targets, and their downstream signaling pathways is crucial for understanding the intricate mechanisms of stroma cell-mediated hyperplasia.
Developing Novel Therapeutic Strategies, How can stroma cells cause hyperplasia
The potential to translate research findings into clinical applications holds immense promise for treating hyperplastic disorders. Developing novel therapeutic strategies that target the stroma cell-mediated mechanisms of hyperplasia could offer a new paradigm for managing these conditions.
“Targeting the stroma cell microenvironment may offer a new avenue for therapeutic intervention in hyperplastic disorders.”
Harnessing the Power of Stroma Cells for Regenerative Medicine
The ability of stroma cells to influence cell growth and differentiation presents an exciting opportunity for regenerative medicine. Exploring the potential of stroma cells to promote tissue regeneration and repair in the context of hyperplastic conditions could lead to innovative therapies that address both the pathological and regenerative aspects of these disorders.
The complex interplay between stroma cells and target cells in hyperplasia offers a wealth of potential therapeutic targets. By understanding the mechanisms underlying stroma cell-mediated hyperplasia, we can develop strategies to modulate this process, potentially leading to new treatments for a range of diseases. Further research is needed to fully elucidate the intricate signaling pathways involved and to translate these findings into clinically relevant therapies.
The potential for targeting stroma cells to control hyperplasia holds immense promise for improving patient outcomes and advancing our understanding of tissue growth and development.
User Queries
What are some examples of diseases where stroma cells contribute to hyperplasia?
Stroma cells play a role in various hyperplastic disorders, including prostate hyperplasia, endometrial hyperplasia, and certain types of cancer.
How do stroma cells influence the proliferation of target cells?
Stroma cells can secrete growth factors and cytokines that bind to receptors on target cells, activating signaling pathways that promote cell division and proliferation.
Can stroma cells contribute to both physiological and pathological hyperplasia?
Yes, stroma cells can contribute to both physiological hyperplasia, such as during wound healing, and pathological hyperplasia, such as in the case of benign prostatic hyperplasia.






