Is the stroma acidic or basic? This question delves into the intricate world of plant cells, specifically the fluid-filled region within chloroplasts known as the stroma. This critical compartment plays a vital role in photosynthesis, the process that fuels life on Earth. Understanding the pH of the stroma is crucial for comprehending the intricate mechanisms that drive this essential process.
The stroma’s pH is not a static value; it fluctuates in response to various factors, including light intensity, environmental conditions, and the plant’s metabolic needs. These fluctuations are tightly regulated, ensuring optimal conditions for the complex enzymatic reactions that occur within the stroma. In this article, we will explore the factors influencing stroma pH, its impact on photosynthesis, and the methods used to measure this critical parameter.
Stroma Definition and Function
The stroma is a vital component of chloroplasts, the organelles responsible for photosynthesis in plant cells. It plays a crucial role in the process of converting light energy into chemical energy, ultimately fueling the growth and development of plants.The stroma, a thick fluid, surrounds the thylakoid membranes within the chloroplast. It is a dynamic environment where numerous biochemical reactions occur, contributing to the overall photosynthetic process.
Stroma Composition and Structure
The stroma is a complex mixture of various components, each contributing to its unique structure and function. The stroma comprises:
- Enzymes: The stroma is rich in enzymes, including those involved in the Calvin cycle, the light-independent reactions of photosynthesis. These enzymes catalyze a series of reactions that convert carbon dioxide into sugars, providing the plant with its primary source of energy.
- DNA and Ribosomes: Chloroplasts possess their own DNA and ribosomes, enabling them to synthesize some of their own proteins. This autonomy allows for efficient regulation of the photosynthetic process within the chloroplast.
- Starch Granules: The stroma stores starch, the primary form of carbohydrate produced by photosynthesis. Starch granules serve as a readily available energy reserve for the plant.
- Other Molecules: The stroma also contains various other molecules, including ions, pigments, and regulatory proteins, all contributing to the intricate processes occurring within the chloroplast.
The stroma’s structure is characterized by its fluidity, allowing for the movement of molecules and enzymes essential for the efficient functioning of photosynthesis. The thylakoid membranes, embedded within the stroma, provide the platform for the light-dependent reactions of photosynthesis, generating ATP and NADPH, which are then utilized in the Calvin cycle within the stroma.
Stroma Function in Photosynthesis
The stroma plays a critical role in the light-independent reactions of photosynthesis, also known as the Calvin cycle. Here, the stroma acts as the central hub for the conversion of carbon dioxide into sugars.The Calvin cycle, occurring within the stroma, involves a series of steps:
- Carbon Fixation: The enzyme RuBisCO catalyzes the fixation of carbon dioxide from the atmosphere, incorporating it into an organic molecule.
- Reduction: The fixed carbon is then reduced using the energy from ATP and NADPH generated during the light-dependent reactions, converting it into a three-carbon sugar.
- Regeneration: The three-carbon sugar is further processed, regenerating the initial molecule for the cycle to continue, ultimately producing glucose, the primary energy source for the plant.
The stroma, therefore, acts as the central processing unit for the Calvin cycle, providing the necessary enzymes, energy carriers, and other molecules required for the efficient conversion of carbon dioxide into sugars, ultimately fueling the plant’s growth and development.
pH and its Importance

The pH scale is a logarithmic measure of the hydrogen ion concentration in a solution. It ranges from 0 to 14, with 0 being the most acidic, 7 being neutral, and 14 being the most basic. Understanding pH is crucial in biological systems because it significantly influences the functioning of various cellular processes.The pH of a solution reflects the concentration of hydrogen ions (H+) and hydroxide ions (OH-) in the solution.
A high concentration of H+ ions results in a low pH, indicating acidity. Conversely, a low concentration of H+ ions and a high concentration of OH- ions result in a high pH, indicating alkalinity.
Impact of pH on Enzyme Activity
Enzymes are biological catalysts that facilitate biochemical reactions in living organisms. They possess optimal pH ranges for their activity. Deviations from these optimal pH levels can lead to reduced enzyme activity or even complete inactivation. The pH can affect the shape and charge of an enzyme, which can disrupt its ability to bind to its substrate and catalyze reactions.
For example, the enzyme pepsin, which breaks down proteins in the stomach, functions optimally at a pH of 2. However, at a neutral pH, pepsin’s activity is significantly reduced.
pH Regulation of Metabolic Processes
pH plays a crucial role in regulating metabolic processes within the stroma. Metabolic reactions often involve the production or consumption of H+ ions, which can significantly impact the pH of the stroma. To maintain optimal pH levels, cells employ various mechanisms, including:
- Buffer Systems: These systems, composed of weak acids and their conjugate bases, act as pH regulators. They absorb or release H+ ions to minimize pH fluctuations.
- Active Transport: Cells can actively transport H+ ions across membranes to maintain pH balance. This process requires energy and involves specialized membrane proteins.
- Metabolic Regulation: Cells can adjust the rate of metabolic reactions that produce or consume H+ ions to control pH levels. For example, the Calvin cycle, a key metabolic pathway in photosynthesis, produces H+ ions. Cells can regulate the rate of this cycle to maintain pH balance.
Maintaining optimal pH levels within the stroma is essential for the proper functioning of metabolic processes. Deviations from the optimal pH range can disrupt enzyme activity, hinder metabolic pathways, and ultimately compromise cell viability.
Factors Influencing Stroma pH
The pH of the stroma, the fluid-filled space within chloroplasts, is a crucial factor in the efficiency of photosynthesis. It’s not static but rather dynamically regulated by a complex interplay of factors. The pH of the stroma is primarily influenced by the concentration of protons (H+). A higher concentration of protons leads to a lower pH (more acidic), while a lower concentration of protons leads to a higher pH (more basic).
Light Intensity and Stroma pH
Light intensity plays a critical role in regulating stroma pH. During photosynthesis, light energy is used to split water molecules, releasing electrons and protons. This process, known as photolysis, increases the concentration of protons in the stroma, leading to a decrease in pH.
The pH of the stroma can change significantly in response to light intensity. Under high light conditions, the pH can drop to as low as 7.5, while in the dark, it can rise to around 8.0.
Increased light intensity enhances the rate of photolysis, leading to a greater influx of protons into the stroma, resulting in a more acidic environment. This acidic environment is essential for the efficient functioning of the Calvin cycle, the process that uses carbon dioxide to produce sugars.
Environmental Conditions and Stroma pH
The pH of the stroma is also influenced by environmental conditions such as temperature and CO2 concentration.
- Temperature: Higher temperatures can increase the rate of photolysis, leading to a more acidic stroma. This is because higher temperatures accelerate the rate of chemical reactions, including those involved in water splitting.
- CO2 Concentration: The concentration of CO2 in the environment also influences stroma pH. Higher CO2 concentrations lead to a decrease in stroma pH. This is because CO2 dissolves in water to form carbonic acid, which releases protons.
Stroma pH and Photosynthesis
The pH of the stroma, the fluid-filled region within chloroplasts, plays a crucial role in the efficiency of photosynthesis. Maintaining an optimal pH within the stroma is essential for the proper functioning of enzymes and the overall process of converting light energy into chemical energy.
pH Gradients and ATP Production
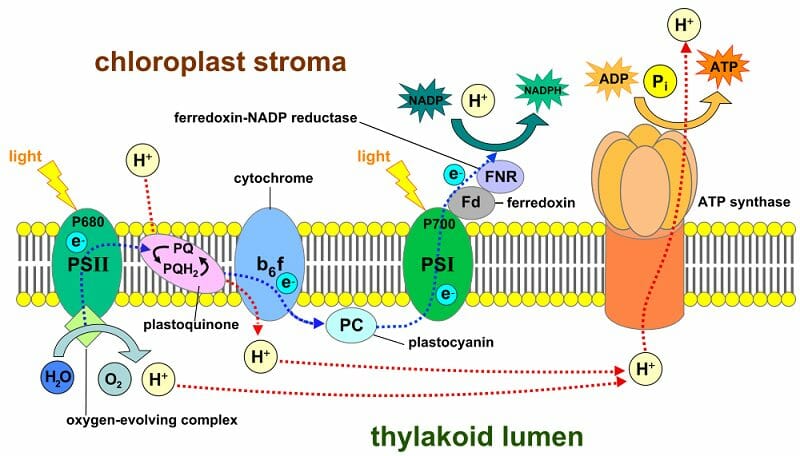
The pH gradient across the thylakoid membrane is a critical factor in ATP production during photosynthesis. The thylakoid membrane encloses a compartment called the thylakoid lumen, which is separated from the stroma by the membrane. During the light-dependent reactions of photosynthesis, protons (H+) are pumped from the stroma into the thylakoid lumen, creating a proton gradient. This gradient represents a difference in pH between the stroma and the lumen, with the lumen being more acidic.
The potential energy stored in this pH gradient is harnessed by ATP synthase, an enzyme embedded in the thylakoid membrane. ATP synthase allows protons to flow back down their concentration gradient from the lumen to the stroma, using the energy released to drive the synthesis of ATP. This process is known as chemiosmosis.
The pH gradient across the thylakoid membrane is essential for ATP production during photosynthesis.
Stroma pH and the Calvin Cycle
The Calvin cycle, the light-independent reactions of photosynthesis, takes place in the stroma. The pH of the stroma influences the activity of enzymes involved in the Calvin cycle. The optimal pH for these enzymes is around 8.0, which is slightly alkaline.A slightly alkaline stroma environment facilitates the following processes in the Calvin cycle:
- Carbon fixation: The enzyme RuBisCO, responsible for fixing carbon dioxide, functions optimally at a slightly alkaline pH. This ensures efficient incorporation of carbon dioxide into organic molecules.
- Regeneration of RuBP: The regeneration of RuBP, the primary carbon acceptor in the Calvin cycle, is also influenced by the pH of the stroma. The enzymes involved in this process function optimally at a slightly alkaline pH.
- Production of sugars: The final step in the Calvin cycle, the production of sugars, is also affected by the pH of the stroma. The enzymes involved in sugar synthesis function optimally at a slightly alkaline pH.
The maintenance of an optimal pH within the stroma is crucial for the efficient functioning of the Calvin cycle and the overall process of photosynthesis.
Methods for Measuring Stroma pH: Is The Stroma Acidic Or Basic
Accurately measuring the pH within the stroma is crucial for understanding the intricate workings of photosynthesis and other metabolic processes within chloroplasts. Several methods have been developed to address this challenge, each with its unique advantages and limitations.The pH of the stroma can be measured using various techniques, each with its own strengths and weaknesses. These techniques allow researchers to delve into the dynamic nature of the stroma’s pH and its impact on chloroplast function.
Direct Measurement Techniques
Direct measurement techniques involve physically accessing the stroma and using pH-sensitive probes or sensors to determine its acidity.
- Microelectrodes: These are tiny, needle-like electrodes inserted directly into the stroma. They are designed to measure the pH of the surrounding environment with high spatial resolution. However, the insertion process can damage the chloroplast and disrupt its normal function.
- pH-sensitive fluorescent dyes: These dyes are introduced into the chloroplast and change their fluorescence intensity based on the surrounding pH. By measuring the fluorescence, researchers can infer the pH of the stroma.
These dyes offer a non-invasive approach, but their sensitivity can be affected by other factors within the chloroplast.
Indirect Measurement Techniques
Indirect methods rely on observing the effects of pH changes on other cellular components or processes.
- NMR spectroscopy: Nuclear Magnetic Resonance (NMR) spectroscopy can be used to detect and quantify specific molecules within the chloroplast. By observing changes in the NMR signals of pH-sensitive molecules, such as phosphate or amino acids, researchers can estimate the stroma pH. This technique is non-invasive and provides information about the overall pH environment, but it lacks the spatial resolution of direct methods.
- Electrochemical techniques: These techniques measure the electrical potential across the chloroplast membrane. This potential is influenced by the pH gradient between the stroma and the thylakoid lumen. By analyzing the electrical potential, researchers can indirectly infer the stroma pH. However, these techniques are complex and require specialized equipment.
Model Stroma Solution Experiment
To understand the principles of pH measurement, a simple experiment can be designed using a model stroma solution.
- Prepare a model stroma solution: This solution should mimic the composition of the actual stroma, including the major ions and buffers present. For example, a solution containing potassium phosphate, magnesium chloride, and sodium bicarbonate could be used.
- Use a pH meter: A standard pH meter can be used to measure the pH of the model stroma solution. Ensure the pH meter is calibrated using standard buffer solutions before making measurements.
- Vary the solution composition: By changing the concentrations of different components in the model stroma solution, you can observe how the pH changes. This will help you understand the role of different components in determining the stroma pH.
- Compare with theoretical predictions: The measured pH can be compared with theoretical predictions based on the solution composition and the dissociation constants of the components. This comparison can help validate the model and identify any discrepancies between the model and reality.
Comparison of Accuracy and Limitations
Each method has its own strengths and weaknesses in terms of accuracy and limitations.
- Direct methods, such as microelectrodes and fluorescent dyes, offer high spatial resolution and can provide accurate pH measurements within the stroma. However, they are invasive and can disrupt the chloroplast’s function.
- Indirect methods, like NMR spectroscopy and electrochemical techniques, are non-invasive but lack the spatial resolution of direct methods. They also rely on indirect measurements and may not be as accurate as direct methods.
- Model stroma solution experiments are valuable for understanding the principles of pH measurement and for testing theoretical predictions. However, they do not reflect the complexity of the actual stroma environment and may not accurately predict the pH in living chloroplasts.
Stroma pH in Different Organisms
The pH of the stroma, the fluid-filled space within chloroplasts, is not uniform across all plant species. Variations in stroma pH are influenced by factors such as the plant’s photosynthetic pathway, environmental conditions, and species-specific adaptations. Understanding these variations provides insights into the diverse strategies plants employ to optimize photosynthesis and thrive in different environments.
Stroma pH in C3, C4, and CAM Plants
The photosynthetic pathways of C3, C4, and CAM plants are known to influence stroma pH. These pathways differ in their strategies for carbon fixation, leading to distinct pH dynamics within the stroma.
- C3 plants, which represent the most common type of photosynthesis, fix carbon dioxide directly through the Calvin cycle. In C3 plants, the stroma pH is generally slightly acidic, ranging from 7.2 to 7.6. This slightly acidic environment supports the optimal activity of enzymes involved in the Calvin cycle.
- C4 plants, adapted to hot and arid environments, utilize a specialized pathway to initially fix carbon dioxide into a four-carbon compound before transporting it to the bundle sheath cells for the Calvin cycle. C4 plants often exhibit a more alkaline stroma pH compared to C3 plants, typically around 7.8 to 8.2. This higher pH may be advantageous for enhancing the activity of enzymes involved in the initial carbon fixation step, particularly in high-temperature conditions.
- CAM plants, adapted to water-stressed environments, fix carbon dioxide at night and store it in the form of malic acid. During the day, they release carbon dioxide for photosynthesis. CAM plants exhibit significant fluctuations in stroma pH, with a more acidic pH during the night (around 7.0 to 7.4) and a more alkaline pH during the day (around 7.8 to 8.2).
These fluctuations reflect the cyclic nature of carbon fixation in CAM plants, where malic acid decarboxylation during the day leads to a temporary increase in pH.
Implications of Stroma pH Differences for Plant Adaptation
The differences in stroma pH between C3, C4, and CAM plants reflect their adaptations to different environmental conditions. For example, the slightly acidic stroma pH in C3 plants may be optimal for the activity of enzymes involved in the Calvin cycle, particularly in temperate environments. The more alkaline stroma pH in C4 plants may provide a competitive advantage in hot and arid environments by enhancing the activity of enzymes involved in the initial carbon fixation step.
Similarly, the fluctuating stroma pH in CAM plants may be crucial for efficient carbon fixation in water-stressed environments, allowing for the accumulation of malic acid at night and its subsequent release during the day for photosynthesis.
The variations in stroma pH across different plant species highlight the intricate relationship between pH and plant adaptation. By fine-tuning the pH of their stroma, plants can optimize the activity of key enzymes involved in photosynthesis and thrive in diverse environments.
Stroma pH and Environmental Stress
Plants, being sessile organisms, are constantly exposed to various environmental stresses that can significantly impact their growth and survival. These stresses can include drought, salinity, extreme temperatures, and nutrient deficiencies. Maintaining a stable internal environment, including a balanced stroma pH, is crucial for plant resilience under these challenging conditions.
Stroma pH Fluctuations under Stress
Stroma pH is tightly regulated under normal conditions. However, environmental stressors can disrupt this delicate balance, leading to significant changes in stroma pH.
Drought Stress
Drought stress, characterized by water scarcity, can drastically affect stroma pH.
- Water loss from plant cells leads to a decrease in turgor pressure, causing stomatal closure and reduced CO 2 uptake.
- This reduced CO 2 availability can lead to a decrease in photosynthetic activity, resulting in a buildup of protons (H +) in the stroma, causing a drop in pH.
- Additionally, drought stress can induce the production of reactive oxygen species (ROS), which can further contribute to acidification of the stroma.
Salinity Stress
Salinity stress, caused by high salt concentrations in the soil, also impacts stroma pH.
- Salt stress can lead to an accumulation of sodium (Na +) ions in the stroma, which can disrupt the pH balance.
- Sodium ions can compete with protons for binding sites on proteins and enzymes, affecting their activity and contributing to pH changes.
- Salt stress can also trigger the production of ROS, further exacerbating the acidification of the stroma.
Stroma pH and Plant Development

The pH of the stroma, the fluid-filled space within chloroplasts, plays a crucial role in regulating various aspects of plant growth and development. This intricate interplay between pH and plant development is essential for proper functioning and survival.Stroma pH acts as a key regulator of plant growth and development, influencing processes such as cell division, elongation, and differentiation. This delicate balance ensures the coordinated growth and development of the plant.
Stroma pH and Flowering Time
Stroma pH is directly linked to the timing of flowering, a critical stage in the plant life cycle. Changes in stroma pH can influence the expression of genes involved in flowering, leading to alterations in the timing of floral initiation and development. This intricate connection ensures the plant flowers at the optimal time for successful reproduction.
The acidic pH of the stroma can promote the expression of genes that initiate flowering, while a more alkaline pH may delay or inhibit flowering.
Stroma pH and Seed Germination, Is the stroma acidic or basic
Stroma pH plays a crucial role in seed germination, the initial stage of plant development. Seed germination is a complex process influenced by several factors, including pH. Changes in stroma pH can impact the activity of enzymes involved in breaking down food reserves within the seed, which is essential for the emerging seedling to obtain nutrients.
Seed germination can be affected by stroma pH, with optimal pH ranges varying among plant species.
The pH of the stroma is a dynamic parameter that plays a crucial role in the intricate dance of photosynthesis. From influencing enzyme activity to regulating metabolic processes, the stroma’s pH is a critical determinant of plant function and survival. As we delve deeper into the mechanisms that govern stroma pH, we gain a greater appreciation for the complex interplay of factors that contribute to the remarkable efficiency of this fundamental process.
Understanding these intricate details is essential for unlocking the secrets of plant life and harnessing its potential for a sustainable future.
FAQ Compilation
What is the typical pH range of the stroma?
The stroma typically has a pH range of 7.2 to 8.0, slightly alkaline.
How does the stroma’s pH compare to other cellular compartments?
The stroma’s pH is generally more alkaline compared to the cytoplasm, which has a pH around 7.0. However, the thylakoid lumen within chloroplasts has a much lower pH, typically around 5.0, due to proton pumping during photosynthesis.
What are the implications of changes in stroma pH for plant health?
Significant changes in stroma pH can disrupt the delicate balance of enzymatic reactions and metabolic processes within the chloroplast. This can negatively impact photosynthesis, growth, and overall plant health.






