What cells make up stroma of thymus – What cells make up the stroma of the thymus? This question delves into the intricate framework that supports the development of T cells, the crucial players in our immune system. The thymus, a small gland located in the chest, serves as the training ground for these immune cells, ensuring they are properly equipped to fight off infections and maintain our health.
Within this gland, the stroma, a network of specialized cells, provides the essential scaffolding and microenvironment for T cell maturation.
This intricate network comprises several key cell types, each playing a distinct role in shaping the destiny of developing T cells. Epithelial cells, the cornerstone of the stroma, create distinct compartments within the thymus, guiding the journey of thymocytes through stages of development. Fibroblasts, the architects of the stroma, contribute to its structural integrity by producing the extracellular matrix. Macrophages, the immune sentinels, patrol the microenvironment, clearing cellular debris and regulating immune responses.
Together, these cells create a dynamic and interactive landscape that ensures the proper development of a diverse repertoire of T cells, capable of recognizing and responding to a vast array of pathogens.
Thymus Structure and Function
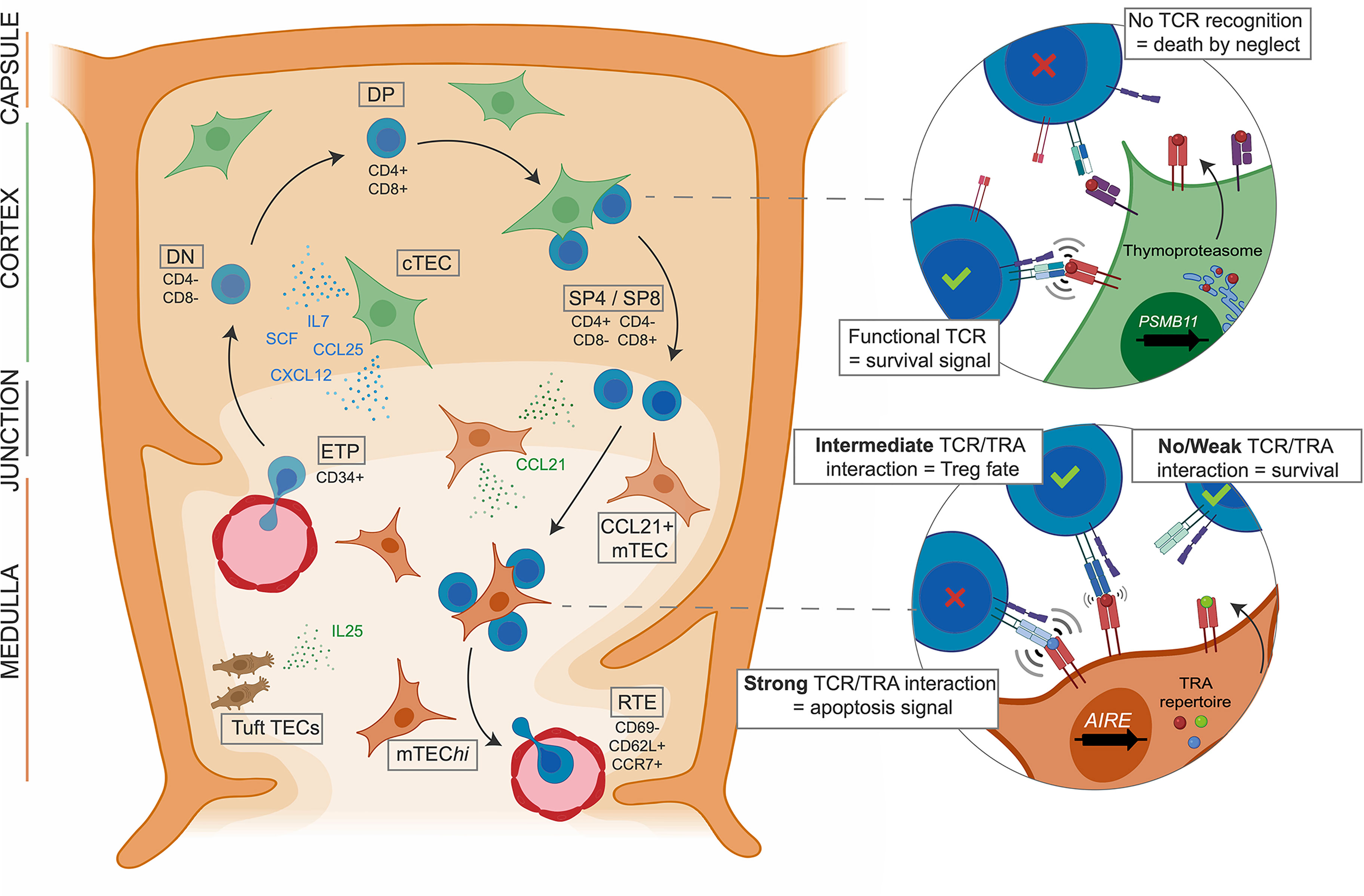
The thymus is a bilobed gland located in the chest, just above the heart. It plays a crucial role in the development and maturation of T lymphocytes, which are essential components of the adaptive immune system.
Thymus Structure
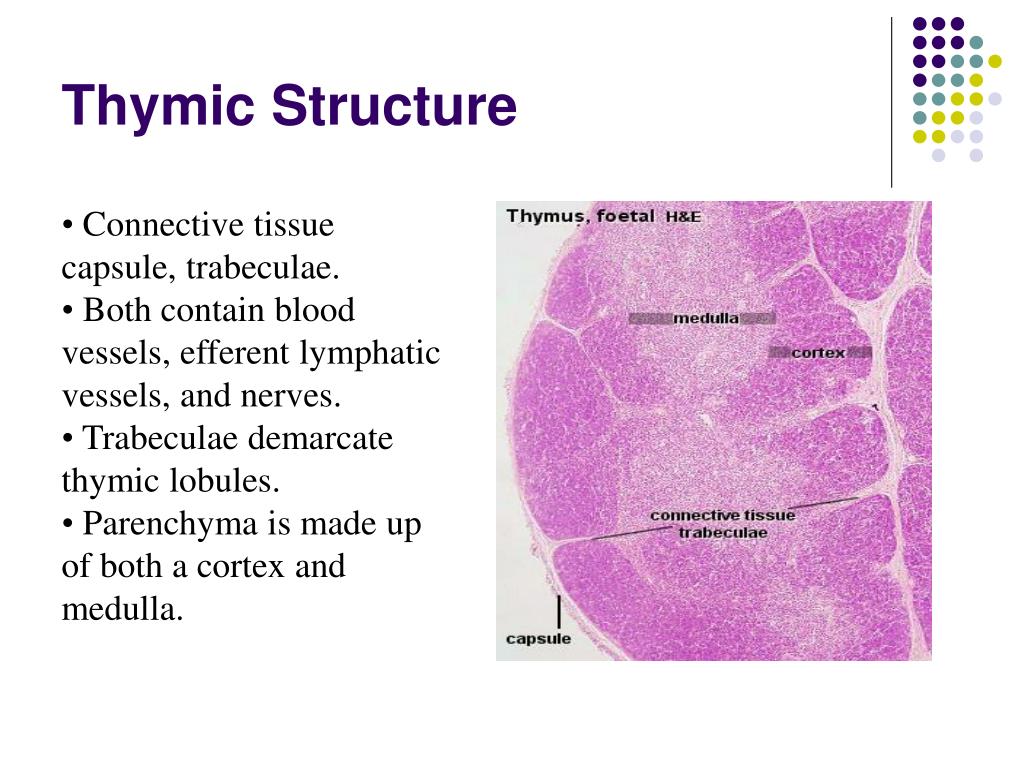
The thymus is divided into two lobes, each of which is further subdivided into lobules. Each lobule is composed of an outer cortex and an inner medulla.
- Cortex: The cortex is the outer region of the lobule and is densely packed with immature T cells called thymocytes. It is also rich in epithelial cells that support thymocyte development.
- Medulla: The medulla is the inner region of the lobule and contains more mature T cells. It is also characterized by the presence of Hassall’s corpuscles, which are concentrically arranged epithelial cells that are thought to play a role in T cell selection.
Thymus Function
The thymus plays a critical role in the development and maturation of T lymphocytes, which are responsible for cell-mediated immunity.
- T Cell Development: Thymocytes undergo a series of developmental stages in the thymus, beginning as immature precursors in the cortex. During this process, they undergo a process of selection and differentiation, which ensures that only T cells with the appropriate receptors are released into the bloodstream.
- T Cell Maturation: Maturation of T cells occurs in the medulla. During this stage, T cells acquire the ability to recognize and respond to specific antigens.
Stroma
The stroma of the thymus gland is the intricate network of non-lymphoid cells that provide structural support and create specialized microenvironments essential for the development and maturation of thymocytes, the immature T cells. This supportive framework plays a critical role in shaping the immune system by orchestrating the selection and differentiation of T cells into functional and self-tolerant lymphocytes.
Stroma Composition and Functions
The thymus stroma is composed of a diverse population of cells, each contributing to the overall function of the organ. These cells include:
- Epithelial Cells: These cells form the structural framework of the thymus, creating distinct compartments within the organ. They are responsible for providing physical support, creating specialized microenvironments, and secreting factors that influence thymocyte development. Epithelial cells are further categorized into:
- Cortical Epithelial Cells (CECs): These cells form the outer layer of the thymus, known as the cortex. They express a variety of molecules, including MHC class I and II, which are crucial for thymocyte selection.
CECs also secrete factors like IL-7, essential for thymocyte proliferation and survival.
- Medullary Epithelial Cells (MECs): These cells are located in the inner region of the thymus, known as the medulla. They express a unique set of molecules, including AIRE (Autoimmune Regulator), which allows them to present self-antigens to thymocytes. This process is critical for establishing central tolerance, preventing autoimmune reactions. MECs also secrete factors like CCL21 and CCL25, which attract mature T cells to the medulla.
- Cortical Epithelial Cells (CECs): These cells form the outer layer of the thymus, known as the cortex. They express a variety of molecules, including MHC class I and II, which are crucial for thymocyte selection.
- Dendritic Cells: These cells are specialized antigen-presenting cells that capture and process antigens from the environment. They migrate from the periphery to the thymus, where they present antigens to thymocytes. This interaction plays a critical role in negative selection, ensuring that only thymocytes that recognize self-antigens with low affinity survive.
- Macrophages: These cells are phagocytic cells that engulf and degrade cellular debris and pathogens. They play a role in maintaining the integrity of the thymus by removing dead cells and pathogens, preventing inflammation.
- Fibroblasts: These cells produce extracellular matrix components, such as collagen and elastin, which provide structural support and maintain the integrity of the thymus.
Stroma’s Role in Thymocyte Development, What cells make up stroma of thymus
The thymus stroma provides a unique microenvironment that is essential for the development and maturation of thymocytes. This microenvironment is characterized by specific cellular interactions, cytokine gradients, and the expression of various molecules that guide thymocyte differentiation and selection.
- Positive Selection: Thymocytes interact with cortical epithelial cells (CECs), which express MHC molecules presenting self-antigens. This interaction allows thymocytes to undergo positive selection, ensuring that only those capable of recognizing self-MHC molecules survive.
- Negative Selection: Thymocytes interact with medullary epithelial cells (MECs) and dendritic cells, which present a wider range of self-antigens. This interaction allows thymocytes to undergo negative selection, eliminating those that recognize self-antigens with high affinity, preventing autoimmune reactions.
- Cytokine Signaling: The stroma secretes a variety of cytokines that influence thymocyte development and differentiation. For example, IL-7 secreted by CECs promotes thymocyte proliferation and survival, while IL-2 secreted by dendritic cells promotes the differentiation of thymocytes into specific T cell subsets.
Cell Types in the Thymus Stroma

The thymus stroma is a complex network of cells that provides a specialized microenvironment essential for the development and maturation of T lymphocytes. This intricate framework is not merely a passive support system; rather, it actively orchestrates the intricate processes of T cell differentiation and selection, ensuring the generation of a diverse and functional T cell repertoire.
Epithelial Cells
Epithelial cells are the primary structural component of the thymus stroma, forming a continuous network that encases the developing T cells. These cells play a pivotal role in guiding T cell development, primarily through the presentation of self-antigens and the secretion of signaling molecules.
Types of Epithelial Cells
- Cortical Epithelial Cells (CECs): Located in the outer cortex of the thymus, CECs are responsible for the initial stages of T cell development, including the expression of key transcription factors and the initiation of TCR rearrangement. These cells present self-antigens to developing thymocytes, contributing to the positive selection process, ensuring the survival of T cells that can recognize self-MHC molecules.
- Medullary Epithelial Cells (MECs): Situated in the inner medulla of the thymus, MECs play a critical role in the later stages of T cell development, including negative selection and the generation of regulatory T cells. These cells present a broader range of self-antigens, eliminating T cells that exhibit strong reactivity to self-peptides, thereby preventing autoimmune reactions.
Fibroblasts
Fibroblasts are another key component of the thymus stroma, contributing to the structural integrity of the organ and the production of the extracellular matrix (ECM). The ECM provides a scaffold for the development of T cells, influencing their migration, proliferation, and differentiation.
Functions of Fibroblasts
- ECM Production: Fibroblasts secrete various ECM components, including collagen, laminin, and fibronectin, which form a network that supports the thymus architecture and provides a physical framework for T cell development.
- Cytokine Secretion: Fibroblasts contribute to the cytokine milieu of the thymus, secreting factors like IL-7, which is essential for the survival and proliferation of early T cell progenitors.
Macrophages
Macrophages are phagocytic cells that reside within the thymus stroma, playing a critical role in the clearance of apoptotic cells and cellular debris. They also contribute to the presentation of self-antigens to developing T cells, further influencing the selection process.
Functions of Macrophages
- Apoptosis Clearance: During T cell development, thymocytes that fail to pass selection checkpoints undergo apoptosis. Macrophages efficiently engulf and degrade these apoptotic cells, preventing the accumulation of cellular debris and maintaining the integrity of the thymus microenvironment.
- Antigen Presentation: Macrophages can process and present self-antigens to developing thymocytes, contributing to the negative selection process. This ensures that T cells with high affinity for self-antigens are eliminated, preventing autoimmunity.
Epithelial Cells
The epithelial cells of the thymus are the key players in the stroma, providing a supportive framework and actively participating in the crucial process of thymocyte development. These cells, distinct from the lymphocytes they nurture, form a network that guides and educates the nascent T cells, ensuring the immune system’s ability to distinguish self from non-self.
Cortical and Medullary Epithelial Cells
The epithelial cells in the thymus are organized into two main types: cortical epithelial cells (cTECs) and medullary epithelial cells (mTECs). Both cell types are essential for thymocyte development, but they play distinct roles in the process.
- Cortical Epithelial Cells (cTECs): Located in the outer cortex of the thymus, cTECs are the primary educators of early thymocytes. They express a diverse array of self-antigens, which are presented to developing thymocytes via major histocompatibility complex (MHC) molecules. This interaction initiates the process of positive selection, where thymocytes that can recognize self-antigens are allowed to survive and progress to the next stage of development.
cTECs also produce signaling molecules that promote thymocyte proliferation and differentiation.
- Medullary Epithelial Cells (mTECs): In the inner medulla of the thymus, mTECs take over the role of educating thymocytes. They express a wider repertoire of self-antigens, including tissue-restricted antigens (TRAs), which are found in specific tissues and organs throughout the body. The presentation of TRAs by mTECs ensures that thymocytes undergo negative selection, eliminating those that react strongly to self-antigens. This process prevents the development of autoreactive T cells that could attack the body’s own tissues.
Hassall’s Corpuscles
Hassall’s corpuscles are unique structures found exclusively in the medulla of the thymus. They are concentrically arranged clusters of mTECs, often described as epithelial cell nests. These structures are thought to play a role in the final stages of thymocyte development, specifically in the induction of T cell tolerance.
- Regulation of T Cell Tolerance: Hassall’s corpuscles are known to produce a variety of factors that contribute to the suppression of autoreactive T cells. These factors include cytokines like IL-10 and TGF-β, which have immunosuppressive effects. Additionally, Hassall’s corpuscles express AIRE (autoimmune regulator), a transcription factor that promotes the expression of TRAs in mTECs, further enhancing the negative selection process.
- Production of Cytokines: Hassall’s corpuscles contribute to the overall microenvironment of the thymus by producing various cytokines, including IL-7, which is essential for thymocyte survival and differentiation. These cytokines help to maintain the integrity of the thymic stroma and support the development of a healthy T cell repertoire.
- Other Functions: While the exact role of Hassall’s corpuscles remains under investigation, studies suggest that they may also be involved in the regulation of thymocyte apoptosis and the maintenance of thymic homeostasis.
Fibroblasts and Macrophages: What Cells Make Up Stroma Of Thymus
Fibroblasts and macrophages, while not directly involved in T cell development, play crucial supporting roles in the thymus stroma. They contribute to the structural integrity and immune regulation of the thymus, creating a suitable environment for T cell maturation.
Fibroblasts and Extracellular Matrix
Fibroblasts, the primary producers of the extracellular matrix (ECM), are essential for maintaining the structural framework of the thymus. The ECM provides a physical support system for epithelial cells, blood vessels, and other stromal components.
- Fibroblasts synthesize various ECM components, including collagen, elastin, fibronectin, and laminin. These proteins form a complex network that provides tensile strength, elasticity, and adhesion properties to the thymus.
- The ECM acts as a scaffold for cell migration and differentiation, guiding the movement of developing T cells through the thymus. It also influences cell signaling and gene expression, contributing to the overall regulation of T cell development.
- The ECM’s composition and organization can vary within different regions of the thymus, reflecting the distinct functional requirements of each compartment.
Macrophages: Phagocytosis and Immune Regulation
Macrophages, the resident phagocytes of the thymus, play a critical role in maintaining the integrity and immune homeostasis of the thymus. They are responsible for removing cellular debris, apoptotic cells, and pathogens, preventing the accumulation of harmful substances within the thymus.
- Macrophages express a wide range of receptors, including Toll-like receptors (TLRs) and Fc receptors, which enable them to recognize and engulf various targets, including bacteria, viruses, and apoptotic cells.
- Phagocytosis by macrophages is crucial for removing apoptotic thymocytes that fail to pass selection checkpoints, ensuring that only mature and functional T cells leave the thymus.
- Macrophages also contribute to immune regulation by presenting antigens to T cells, promoting the development of tolerance to self-antigens and preventing autoimmune reactions.
Interactions Between Stromal Cells
Epithelial cells, fibroblasts, and macrophages interact dynamically to maintain the thymus microenvironment. These interactions are essential for the proper development and function of the thymus.
- Epithelial cells secrete factors that influence fibroblast activity, regulating ECM production and organization.
- Fibroblasts, in turn, provide structural support for epithelial cells and create a suitable environment for macrophage recruitment and activation.
- Macrophages, through their phagocytic and antigen-presenting activities, contribute to the removal of apoptotic cells and the regulation of T cell development, influencing the overall immune response within the thymus.
Stroma and Thymocyte Development
The thymus, a primary lymphoid organ, provides a unique microenvironment for the development of T lymphocytes (thymocytes) from immature progenitors into mature, functional T cells. This intricate process is tightly regulated by the stromal cells that constitute the thymic framework. The stromal cells, including epithelial cells, fibroblasts, and macrophages, play crucial roles in guiding thymocyte differentiation, selection, and maturation.
Stages of T Cell Development
The development of T cells within the thymus involves a series of distinct stages, each characterized by specific molecular markers and functional capabilities. The stromal cells provide essential cues at each stage, influencing thymocyte fate decisions and shaping the T cell repertoire.
- Double-Negative (DN) Stage: Immature thymocytes entering the thymus lack both CD4 and CD8 co-receptors, hence the designation “double-negative” (DN). These DN thymocytes undergo a series of developmental stages within the thymic cortex, driven by interactions with cortical epithelial cells.
- DN1 (CD44+CD25 –): This initial stage involves proliferation and commitment to the T cell lineage.
- DN2 (CD44+CD25 +): DN2 thymocytes undergo β-selection, where they rearrange their T cell receptor (TCR) β-chain genes.
- DN3 (CD44–CD25 +): Successful TCRβ-chain rearrangement leads to pre-TCR expression, signaling for further proliferation and differentiation.
- DN4 (CD44–CD25 –): DN4 thymocytes complete TCRα-chain rearrangement and express a functional αβ TCR, transitioning to the double-positive (DP) stage.
- Double-Positive (DP) Stage: DP thymocytes express both CD4 and CD8 co-receptors. They migrate to the thymic medulla, where they encounter medullary epithelial cells, dendritic cells, and macrophages. These interactions play a critical role in positive and negative selection.
- Single-Positive (SP) Stage: Following selection, DP thymocytes differentiate into either CD4 + or CD8 + single-positive (SP) T cells. This fate decision is determined by the TCR’s affinity for self-MHC molecules.
Molecular Interactions
The development of thymocytes is orchestrated by a complex interplay of molecular signals exchanged between thymocytes and stromal cells. These interactions are essential for guiding thymocyte differentiation, selection, and ultimately, the generation of a diverse T cell repertoire.
- Cytokine Signaling: Stromal cells produce a variety of cytokines, such as IL-7, IL-15, and TNF-α, that regulate thymocyte proliferation, survival, and differentiation. For instance, IL-7 is crucial for the survival of early DN thymocytes.
- Notch Signaling: Notch signaling is critical for thymocyte development, particularly in the DN stages. Cortical epithelial cells express Notch ligands, which interact with Notch receptors on thymocytes. This interaction promotes thymocyte survival, proliferation, and differentiation.
- TCR Signaling: The interaction of the TCR with self-MHC molecules on stromal cells is central to both positive and negative selection.
- Positive Selection: Weak interactions between the TCR and self-MHC molecules on cortical epithelial cells promote the survival of thymocytes that can recognize self-MHC. This ensures that the T cell repertoire can respond to foreign antigens presented by self-MHC.
- Negative Selection: Strong interactions between the TCR and self-MHC molecules on medullary epithelial cells lead to the elimination of thymocytes that recognize self-antigens with high affinity. This process prevents the development of autoreactive T cells that could cause autoimmune diseases.
Contribution of Stroma to T Cell Diversity
The thymus stroma plays a critical role in generating a diverse repertoire of T cells. This diversity is essential for the immune system to effectively respond to a wide range of pathogens.
- Genetic Rearrangements: The thymus provides an environment where thymocytes undergo V(D)J recombination, a process that randomly rearranges gene segments encoding the TCR. This generates a vast array of TCRs with different specificities.
- Selection Processes: Positive and negative selection ensure that only T cells that can recognize self-MHC molecules and that do not recognize self-antigens with high affinity are allowed to mature. This selection process, influenced by the stromal cells, shapes the T cell repertoire, ensuring both self-tolerance and responsiveness to foreign antigens.
- Medullary Microenvironment: The medullary epithelial cells express a wide variety of self-antigens, including tissue-restricted antigens (TRAs). These TRAs are expressed in peripheral tissues but not normally in the thymus. Exposure to TRAs in the medulla allows for the deletion of potentially autoreactive T cells, contributing to self-tolerance.
The thymus stroma, with its diverse cast of cellular players, orchestrates the intricate process of T cell development. This remarkable microenvironment ensures that the immune system is armed with a diverse and robust army of T cells, ready to defend our bodies against a myriad of threats. Understanding the cellular composition and interactions within the thymus stroma is essential for comprehending the complexities of immune development and for exploring potential therapeutic strategies for immune disorders.
FAQs
What is the difference between cortical and medullary epithelial cells?
Cortical epithelial cells are found in the outer region of the thymus and are involved in early T cell development, while medullary epithelial cells are located in the inner region and play a role in T cell selection and tolerance.
What are Hassall’s corpuscles and what is their function?
Hassall’s corpuscles are specialized structures found in the medullary stroma of the thymus. Their exact function is still being investigated, but they are thought to play a role in T cell maturation and immune regulation.
How do fibroblasts contribute to the thymus microenvironment?
Fibroblasts produce extracellular matrix components, which provide structural support and help create the appropriate microenvironment for thymocyte development.
What are the key functions of macrophages in the thymus?
Macrophages are phagocytic cells that remove cellular debris and apoptotic thymocytes. They also play a role in immune regulation by presenting antigens to T cells and secreting cytokines.






