What is gland to stroma ratio? It’s a seemingly simple concept, yet it holds profound implications for understanding the intricacies of tissue health and disease. This ratio, the balance between glandular and stromal components within a tissue, serves as a silent language, whispering vital information about the underlying processes at play.
From the bustling city of cells within a healthy gland to the silent whispers of a tumor’s growth, the gland to stroma ratio paints a vivid picture of the tissue’s landscape. In this journey, we delve into the methods for deciphering this ratio, the factors that influence its fluctuations, and its profound applications in diverse fields, from cancer diagnosis to tissue engineering.
Introduction to Gland to Stroma Ratio

Within the intricate tapestry of human tissues, a delicate balance exists between functional units, known as glands, and the supportive framework that surrounds them, referred to as stroma. The gland to stroma ratio, a quantitative measure of this balance, serves as a vital tool in understanding the structure and function of tissues, particularly in the realms of pathology, oncology, and regenerative medicine.The gland to stroma ratio quantifies the relative proportions of glandular and stromal components within a tissue sample.
It provides valuable insights into the architectural organization of the tissue, reflecting the interplay between glandular activity and the surrounding supportive structures.
Significance of Gland to Stroma Ratio
The gland to stroma ratio holds significant implications across various fields, revealing intricate connections between tissue structure, function, and disease progression.
- Pathology: In pathology, the gland to stroma ratio plays a crucial role in diagnosing and classifying diseases. For example, in breast cancer, a high gland to stroma ratio is often associated with a more aggressive tumor, while a low ratio may indicate a less aggressive form. Similarly, in prostate cancer, a high gland to stroma ratio can be indicative of tumor progression and increased risk of metastasis.
- Oncology: In oncology, the gland to stroma ratio serves as a prognostic marker, helping to predict the course of the disease and guide treatment strategies. By understanding the balance between glandular and stromal components, oncologists can assess the potential for tumor growth, spread, and response to therapy. For instance, a high gland to stroma ratio in a tumor may indicate a more aggressive growth pattern, requiring more aggressive treatment interventions.
- Regenerative Medicine: In regenerative medicine, the gland to stroma ratio is a key factor in assessing the success of tissue regeneration. By understanding the balance between glandular and stromal components, researchers can optimize conditions for tissue repair and regeneration. For example, in the development of engineered tissues, a carefully controlled gland to stroma ratio is essential for achieving optimal functionality and integration with the surrounding host tissue.
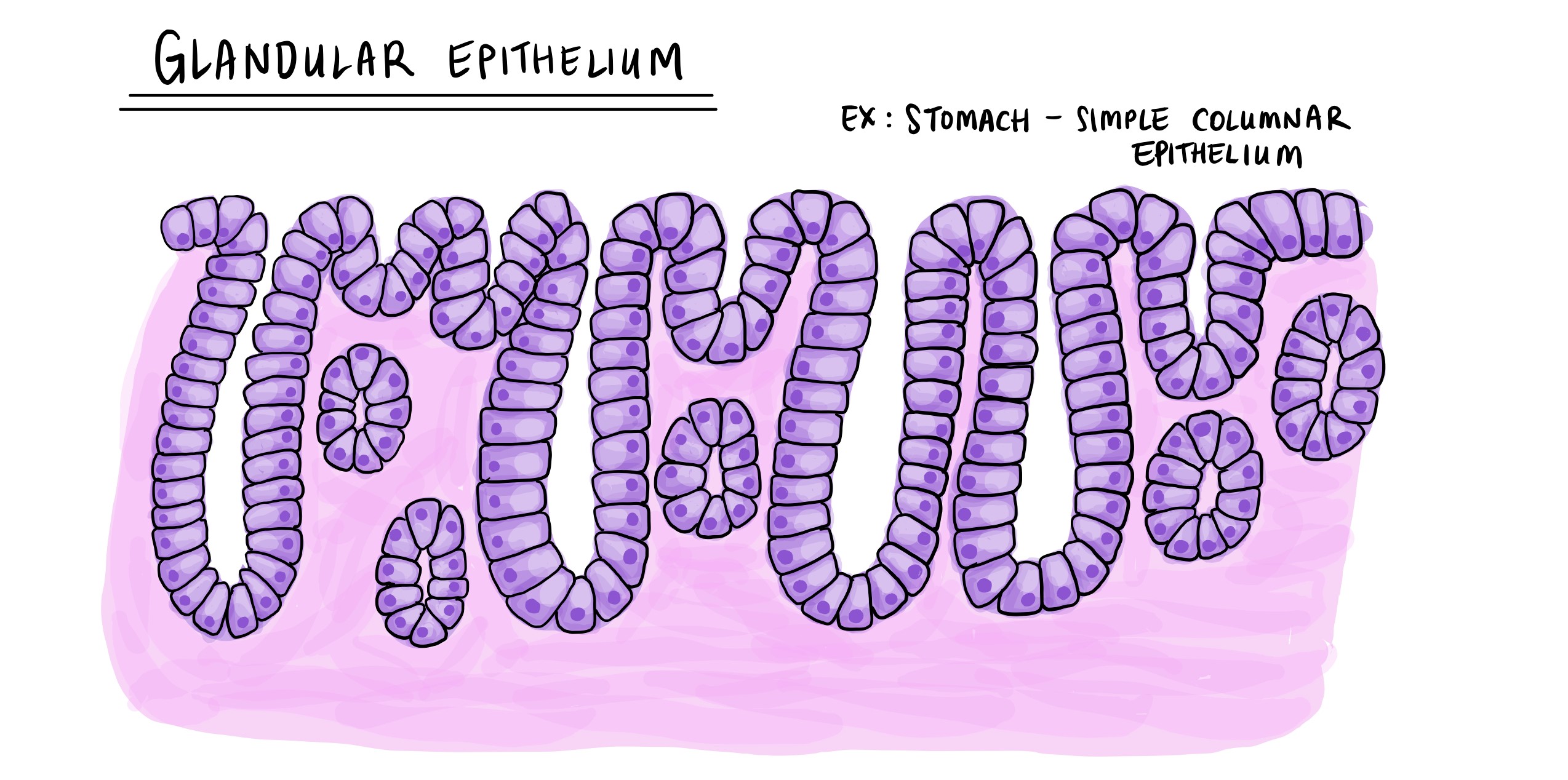
Examples of Glands and Their Corresponding Stromal Tissues
The gland to stroma ratio is a versatile tool applicable to a wide range of tissues, each characterized by specific glandular and stromal components.
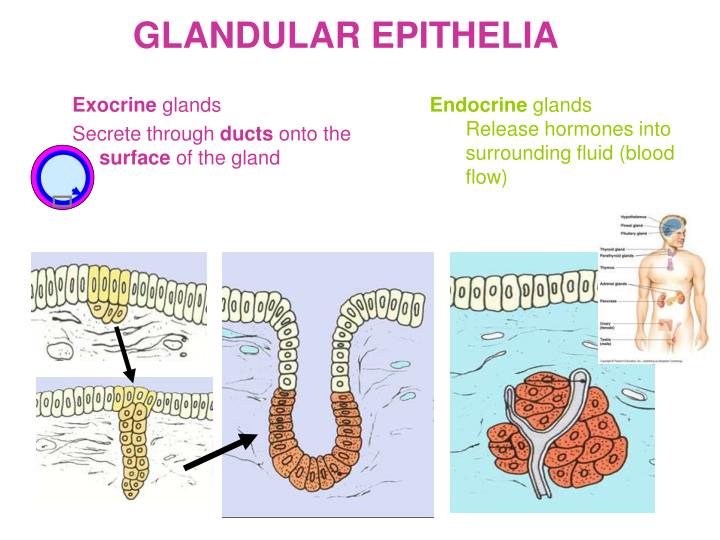
- Endocrine Glands: Endocrine glands, such as the thyroid gland, are responsible for secreting hormones directly into the bloodstream. The stroma of endocrine glands typically consists of connective tissue, blood vessels, and nerves, providing support and facilitating hormone release. The gland to stroma ratio in endocrine tissues can vary depending on the specific gland and its activity level.
- Exocrine Glands: Exocrine glands, such as salivary glands, secrete their products through ducts into specific locations. The stroma of exocrine glands often includes connective tissue, smooth muscle, and ducts, supporting the gland’s structure and facilitating product delivery. The gland to stroma ratio in exocrine tissues can be influenced by factors such as the gland’s size, activity, and age.
- Epithelial Glands: Epithelial glands, such as the sweat glands, are found in epithelial tissues and play roles in secretion and absorption. The stroma of epithelial glands typically comprises connective tissue, providing support and anchoring the glands to the surrounding tissues. The gland to stroma ratio in epithelial tissues can vary depending on the specific location and function of the glands.
Methods for Determining Gland to Stroma Ratio
The determination of gland to stroma ratio is crucial for understanding the structure and function of various tissues, particularly in the context of pathology and cancer research. This ratio can provide insights into the growth and development of tissues, as well as the potential for disease progression. Various methods have been developed to quantify this ratio, each offering unique advantages and limitations.
Microscopic Analysis
Microscopic analysis is a cornerstone of gland to stroma ratio determination. It involves the examination of histological sections, thin slices of tissue that are stained to highlight different cellular components. This technique allows for the visualization and quantification of both glandular and stromal elements.
Histological sections are stained with specific dyes to differentiate between glandular and stromal components. For instance, hematoxylin and eosin (H&E) staining is a widely used technique that stains nuclei blue and cytoplasm pink, allowing for the identification of different cell types.
Image analysis software plays a crucial role in the quantification process. These programs can automatically identify and measure the areas occupied by glandular and stromal components within the histological sections. This automated approach minimizes subjectivity and enhances accuracy in determining the gland to stroma ratio.
Immunohistochemistry
Immunohistochemistry is a powerful technique that uses antibodies to specifically target and label particular proteins within tissue sections. This method allows for the identification and quantification of glandular and stromal components based on the expression of specific markers.
For example, antibodies targeting cytokeratins can be used to identify glandular epithelial cells, while antibodies targeting vimentin can be used to identify stromal cells.
The staining patterns obtained through immunohistochemistry provide valuable information about the distribution and density of glandular and stromal components. This information can be further analyzed to calculate the gland to stroma ratio, offering a more precise and specific assessment of tissue composition.
Flow Cytometry
Flow cytometry is a technique that analyzes individual cells based on their physical and biochemical properties. This method involves suspending cells in a fluid stream and passing them through a laser beam. The scattered light and emitted fluorescence are then detected and analyzed, allowing for the differentiation of cell populations based on specific markers.
Flow cytometry can be used to identify and quantify glandular and stromal cells based on the expression of specific surface markers. For example, cells expressing epithelial cell adhesion molecule (EpCAM) can be identified as glandular epithelial cells, while cells expressing CD34 can be identified as stromal cells.
Flow cytometry provides a quantitative analysis of cell populations, allowing for the determination of the gland to stroma ratio based on the relative proportions of glandular and stromal cells. This method offers a high-throughput approach for analyzing large numbers of cells, making it suitable for studies involving tissue heterogeneity.
Factors Influencing Gland to Stroma Ratio
The gland to stroma ratio, a crucial parameter in assessing tissue health and function, is not static. It’s a dynamic measure that can fluctuate due to a myriad of factors, reflecting the intricate interplay of cellular processes within the tissue. Understanding these influences is essential for accurate interpretation of the ratio and for guiding clinical decision-making.
Disease State
The presence of disease can significantly alter the gland to stroma ratio. Different diseases can manifest with distinct changes in the ratio, providing valuable insights into disease progression and severity.
- Benign Prostatic Hyperplasia (BPH): In BPH, the prostate gland undergoes an enlargement, leading to an increase in the glandular component, resulting in a higher gland to stroma ratio. This increase is primarily due to the proliferation of epithelial cells lining the glands.
- Prostate Cancer: Conversely, prostate cancer often exhibits a lower gland to stroma ratio. This reduction is attributed to the infiltration of cancerous cells, which can replace normal glandular tissue and disrupt the tissue architecture. The presence of dense stroma, a fibrous connective tissue, is also characteristic of prostate cancer.
- Breast Cancer: In breast cancer, the gland to stroma ratio can be either increased or decreased depending on the specific subtype. Some breast cancers, like ductal carcinoma in situ (DCIS), may show an increased ratio due to the proliferation of malignant cells within the ducts. However, invasive breast cancers often exhibit a decreased ratio as cancerous cells invade and replace normal glandular tissue.
Age, What is gland to stroma ratio
The aging process can subtly but significantly influence the gland to stroma ratio.
- Prostate: With age, the prostate gland often undergoes changes, including an increase in stromal tissue and a decrease in glandular tissue. This shift leads to a lower gland to stroma ratio in older men. This age-related change in the prostate is a significant contributor to BPH.
- Breast: Similar to the prostate, the breast tissue also experiences age-related changes. Postmenopausal women often exhibit a higher stromal component compared to premenopausal women, leading to a lower gland to stroma ratio. These changes are influenced by hormonal fluctuations associated with menopause.
Hormonal Status
Hormones play a crucial role in regulating tissue growth and development, and consequently, they can influence the gland to stroma ratio.
- Androgens: Androgens, particularly testosterone, are known to stimulate prostate gland growth. In men with high testosterone levels, the gland to stroma ratio may be elevated. Conversely, low testosterone levels can lead to a decrease in the ratio.
- Estrogens: Estrogens, primarily produced by the ovaries in women, can also influence the gland to stroma ratio in breast tissue. High estrogen levels, especially during pregnancy or hormone replacement therapy, can stimulate breast gland growth, leading to an increased ratio. Conversely, low estrogen levels, as seen during menopause, can contribute to a decrease in the ratio.
Treatment Modalities
Various treatment modalities, including surgery, chemotherapy, and radiation therapy, can impact the gland to stroma ratio.
- Surgery: Surgical removal of a portion of the gland, as in a prostatectomy, will directly reduce the gland to stroma ratio. This reduction is due to the physical removal of glandular tissue.
- Chemotherapy: Chemotherapy, a systemic treatment for cancer, can affect the gland to stroma ratio by targeting rapidly dividing cells, including both cancerous and normal glandular cells. This can lead to a decrease in the ratio as glandular tissue is destroyed.
- Radiation Therapy: Radiation therapy, a localized treatment for cancer, can also impact the gland to stroma ratio by damaging both glandular and stromal tissue. The effect on the ratio depends on the specific radiation dose and the area targeted.
Clinical Applications of Gland to Stroma Ratio
The gland-to-stroma ratio, a measure of the relative proportion of glandular tissue to connective tissue, plays a crucial role in various clinical settings. Its application extends beyond basic tissue analysis, offering valuable insights into disease diagnosis, prognosis, and treatment monitoring.
Cancer Diagnosis and Prognosis
The gland-to-stroma ratio has emerged as a valuable tool in cancer diagnosis and prognosis, particularly in the context of breast cancer. A lower gland-to-stroma ratio, indicating a higher proportion of stromal tissue, is often associated with a more aggressive tumor behavior and poorer prognosis. This association can be attributed to several factors:
A lower gland-to-stroma ratio may indicate a more aggressive tumor behavior due to increased stromal cell activity, which can contribute to tumor invasion and metastasis.
A higher proportion of stromal tissue can also limit the effectiveness of certain cancer treatments, such as chemotherapy, by hindering drug penetration.
Studies have shown that patients with breast cancer exhibiting a lower gland-to-stroma ratio tend to have a higher risk of recurrence and a lower overall survival rate. Conversely, a higher gland-to-stroma ratio is often associated with a more favorable prognosis.
Monitoring Disease Progression
The gland-to-stroma ratio can also be used to monitor disease progression and assess the effectiveness of treatment. Changes in the ratio over time can provide valuable information about the response to therapy. For example, a decrease in the ratio after treatment could indicate tumor shrinkage and a positive response to therapy.
The gland-to-stroma ratio can serve as a valuable indicator of tumor response to treatment, allowing clinicians to monitor disease progression and adjust treatment strategies accordingly.
Tissue Engineering and Regeneration
The gland-to-stroma ratio also holds promise in the field of tissue engineering and regeneration. By controlling the ratio of glandular and stromal cells, researchers can create artificial tissues that more closely resemble native tissues.
The gland-to-stroma ratio plays a critical role in guiding tissue regeneration, as the balance between glandular and stromal cells is crucial for the formation of functional tissues.
For example, researchers are investigating the use of gland-to-stroma ratios to create engineered tissues for transplantation, such as skin grafts, cartilage replacements, and pancreatic islets.
Future Directions in Gland to Stroma Ratio Research: What Is Gland To Stroma Ratio
The exploration of gland to stroma ratio has yielded significant insights into various diseases, particularly in the realm of cancer. However, the journey of unraveling the complexities of this ratio is far from over. The future holds exciting possibilities for research in this field, with a focus on refining existing methods, uncovering new biomarkers, and leveraging personalized medicine approaches.
Development of Novel Imaging Techniques
Advancements in imaging technology are crucial for enhancing the accuracy and precision of gland to stroma ratio determination. Current methods, while valuable, often rely on subjective assessments, leading to potential variability in interpretations.
- Developing advanced imaging techniques, such as multiphoton microscopy and super-resolution microscopy, can provide higher resolution and deeper tissue penetration, enabling more precise quantification of glandular and stromal components.
- Quantitative image analysis algorithms can be integrated with these imaging techniques to automate the process of gland to stroma ratio determination, reducing inter-observer variability and improving consistency.
Identification of New Biomarkers
The identification of novel biomarkers associated with gland to stroma ratio holds immense potential for improving diagnostic and prognostic capabilities.
- Investigating the molecular pathways involved in gland to stroma ratio alterations can lead to the discovery of specific protein markers or gene expression signatures that reflect the ratio and its clinical significance.
- Liquid biopsies, which analyze circulating tumor cells or tumor DNA in bodily fluids, could potentially provide non-invasive means of monitoring gland to stroma ratio changes over time, facilitating early detection and personalized treatment strategies.
Personalized Medicine Applications
The concept of personalized medicine, tailoring treatments to individual patients based on their unique characteristics, is gaining momentum. Gland to stroma ratio holds significant potential for enhancing personalized medicine approaches.
- By understanding the specific gland to stroma ratio in a patient’s tumor, clinicians can select the most appropriate treatment regimen, whether it involves surgery, chemotherapy, or radiation therapy, leading to improved outcomes and reduced side effects.
- Targeted therapies, designed to specifically target molecular alterations associated with gland to stroma ratio, could offer more effective and less toxic treatment options for patients with specific tumor characteristics.
The gland to stroma ratio, a seemingly simple measure, reveals a complex world of tissue dynamics. It is a powerful tool for unraveling the mysteries of disease, guiding treatment strategies, and pushing the boundaries of regenerative medicine. As we continue to explore its depths, we uncover a deeper understanding of the intricate dance between cells, tissues, and the delicate balance of life itself.
Essential FAQs
What are some examples of glands and their corresponding stromal tissues?
Glands can be classified as exocrine or endocrine, each with distinct stromal components. Exocrine glands, such as salivary glands, have a supporting stroma composed of connective tissue, blood vessels, and nerves. Endocrine glands, like the thyroid, are typically surrounded by a capsule of connective tissue and have a rich vascular network.
How is gland to stroma ratio used in personalized medicine?
Personalized medicine aims to tailor treatments to individual patients based on their unique characteristics. Gland to stroma ratio can play a crucial role in this approach by providing insights into a patient’s tumor biology and response to therapy. This information can help guide treatment decisions and optimize outcomes.






