Was leitet Strom und was nicht, a question that probes the very essence of electricity, leads us on a fascinating journey into the world of conductors and insulators. Imagine a world where electricity could flow through everything – chaos! But thankfully, nature has provided us with materials that either readily allow or fiercely resist the flow of electrons, shaping the electrical systems that power our lives.
From the copper wires that carry power to our homes to the plastic casings that protect us from electric shocks, conductors and insulators play crucial roles in our technological world. Understanding their properties and how they interact with electricity is essential for comprehending the electrical systems that surround us.
What Conducts Electricity
/examples-of-electrical-conductors-and-insulators-608315_v3-5b609152c9e77c004f6e8892.png)
Electricity is a fundamental force of nature that plays a crucial role in our modern world. It powers our homes, businesses, and transportation systems, and it’s essential for countless technologies. Understanding how electricity flows is crucial for comprehending its applications and limitations. This section explores the concept of electrical conductivity and the materials that excel in conducting electricity.
Materials That Conduct Electricity
Electrical conductivity is a material’s ability to allow the flow of electric current. This flow is facilitated by the movement of electrically charged particles, primarily electrons. Materials with high electrical conductivity allow electrons to move freely, enabling the passage of electric current. Here are some common materials that are good conductors of electricity:
- Metals: Metals are excellent conductors of electricity due to the presence of free electrons in their atomic structure. These electrons are not tightly bound to individual atoms and can easily move throughout the material, facilitating the flow of electric current. Common examples include copper, silver, gold, aluminum, and iron.
- Graphite: Graphite is a form of carbon with a layered structure. Within each layer, electrons are delocalized and can move freely, making graphite a good conductor of electricity.
- Electrolytes: Electrolytes are solutions or molten substances containing ions. These ions can move freely and carry electric charge, enabling the flow of electricity. Examples include saltwater, battery acid, and molten salts.
- Plasma: Plasma is a superheated gas where electrons are stripped from atoms, creating a mixture of ions and free electrons. The presence of free electrons makes plasma an excellent conductor of electricity.
The Role of Free Electrons
The ability of a material to conduct electricity is directly linked to the presence of free electrons. In metals, the outer electrons of atoms are loosely bound and can easily detach from their parent atoms, becoming free electrons. These free electrons are not confined to specific atoms and can move throughout the material, forming an “electron sea.”
Atomic Structure of Conductors
The atomic structure of conductors plays a crucial role in their ability to conduct electricity. Metals, for instance, have a unique atomic arrangement where the outermost electrons are weakly bound to the nucleus. These loosely bound electrons are readily available to move freely, creating an “electron sea” that facilitates electrical conductivity.
Table of Electrical Conductivity
The following table summarizes the electrical conductivity of various materials, their applications, and some common examples:
| Material | Conductivity (Siemens/meter) | Applications | Examples |
|---|---|---|---|
| Silver | 63 x 106 | Electronics, jewelry, mirrors | Silver coins, silver wires |
| Copper | 59 x 106 | Electrical wiring, plumbing | Copper wires, copper pipes |
| Gold | 45 x 106 | Electronics, jewelry | Gold plating, gold coins |
| Aluminum | 38 x 106 | Construction, aircraft | Aluminum foil, aluminum cans |
| Iron | 10 x 106 | Steel production, construction | Steel beams, iron pipes |
| Graphite | 1 x 106 | Batteries, pencils, electrodes | Graphite batteries, pencil lead |
| Saltwater | 5 x 10-2 | Electrolysis, battery solutions | Seawater, salt solutions |
What Does Not Conduct Electricity

Electricity is a fundamental force that powers our modern world. It flows through wires, circuits, and devices, enabling everything from lighting our homes to running our computers. However, not everything conducts electricity. Some materials, known as insulators, resist the flow of electric current. Understanding the properties of insulators is crucial for designing safe and efficient electrical systems.
Insulators and Electrical Conductivity
An insulator is a material that strongly resists the flow of electric current. This resistance arises from the unique atomic structure of insulators. Unlike conductors, which have loosely bound electrons that can easily move, insulators have tightly bound electrons that are difficult to dislodge. This tight binding prevents the free movement of electrons, which is essential for electrical conductivity.
Examples of Common Insulating Materials
Insulators are ubiquitous in our daily lives, protecting us from electrical hazards and ensuring the safe operation of electrical devices. Here are some common examples:
- Rubber: Rubber is an excellent insulator commonly used in electrical cords, gloves, and mats. Its insulating properties stem from its long, chain-like molecules that tightly bind electrons, preventing their movement.
- Glass: Glass is another widely used insulator, found in light bulbs, windows, and electrical insulators. Its tightly packed atomic structure restricts electron movement, making it an effective barrier to electricity.
- Plastic: Plastics are versatile insulators used in a wide range of applications, including electrical casings, insulation, and packaging. The diverse chemical compositions of different plastics provide varying levels of insulating properties.
Properties of Insulators
Insulators possess several key properties that contribute to their ability to block the flow of electricity:
- High Resistance: Insulators exhibit a high electrical resistance, meaning they oppose the flow of electric current. This resistance is due to the tightly bound electrons that resist movement.
- Low Conductivity: Insulators have low electrical conductivity, meaning they allow very little electric current to flow through them. This low conductivity is a direct result of their high resistance.
- Energy Gap: Insulators have a large energy gap between their valence band and conduction band. This gap represents the amount of energy required to excite an electron from its bound state to a free state, enabling it to conduct electricity. The large energy gap in insulators makes it difficult for electrons to become free, preventing current flow.
Atomic Structure of Insulators
The atomic structure of insulators is fundamentally different from that of conductors. In insulators, the outermost electrons, known as valence electrons, are tightly bound to the nucleus. These electrons are not easily freed from their atoms, preventing the flow of electric current.
In contrast, conductors have loosely bound valence electrons that can easily move from atom to atom, enabling the flow of electricity.
Flowchart Illustrating the Differences Between Conductors and Insulators
Here is a flowchart illustrating the key differences between conductors and insulators:
- Start: Material
- Question: Does the material have loosely bound electrons?
- Yes: Conductor
- No: Insulator
Factors Affecting Conductivity
The conductivity of a material, its ability to conduct electricity, is not a fixed property. It can be influenced by various factors, including temperature, impurities, and pressure. Understanding these factors is crucial for optimizing the use of conductive materials in different applications.
Temperature’s Influence on Conductivity
Temperature significantly impacts the conductivity of materials, especially conductors. As temperature increases, the atoms within a conductor vibrate more vigorously. This increased vibration leads to more frequent collisions between electrons and atoms, hindering the free flow of electrons. Consequently, the conductivity of most conductors decreases with increasing temperature.
Impact of Impurities on Conductivity
Impurities, even in small amounts, can dramatically alter the conductivity of a material. The presence of impurities disrupts the regular arrangement of atoms in the material’s structure, creating imperfections that act as obstacles for electron flow. These obstacles scatter electrons, reducing their overall mobility and thus decreasing conductivity.
Pressure’s Effect on Conductivity
Pressure plays a significant role in the conductivity of solids and liquids. In solids, increased pressure can force atoms closer together, reducing the spacing between them. This tighter packing can enhance the overlap of electron orbitals, facilitating electron movement and increasing conductivity. In liquids, pressure can affect the density and mobility of ions, which are responsible for electrical conduction.
Increased pressure can lead to higher ion density and mobility, resulting in increased conductivity.
Factors Affecting Conductivity Table
| Factor | Effect on Conductivity | Examples | |
|---|---|---|---|
| Temperature | Increased temperature generally reduces conductivity in most conductors. | – Copper wire’s conductivity decreases with increasing temperature.
| |
| Impurities | Impurities typically decrease conductivity by scattering electrons. | – Adding impurities to silicon can reduce its conductivity, making it suitable for creating resistors.
| |
| Pressure | Increased pressure generally increases conductivity in solids and liquids. | – Applying pressure to a metal wire can enhance its conductivity. | – Increasing pressure on a liquid electrolyte can increase its conductivity. |
Applications of Conductors and Insulators: Was Leitet Strom Und Was Nicht
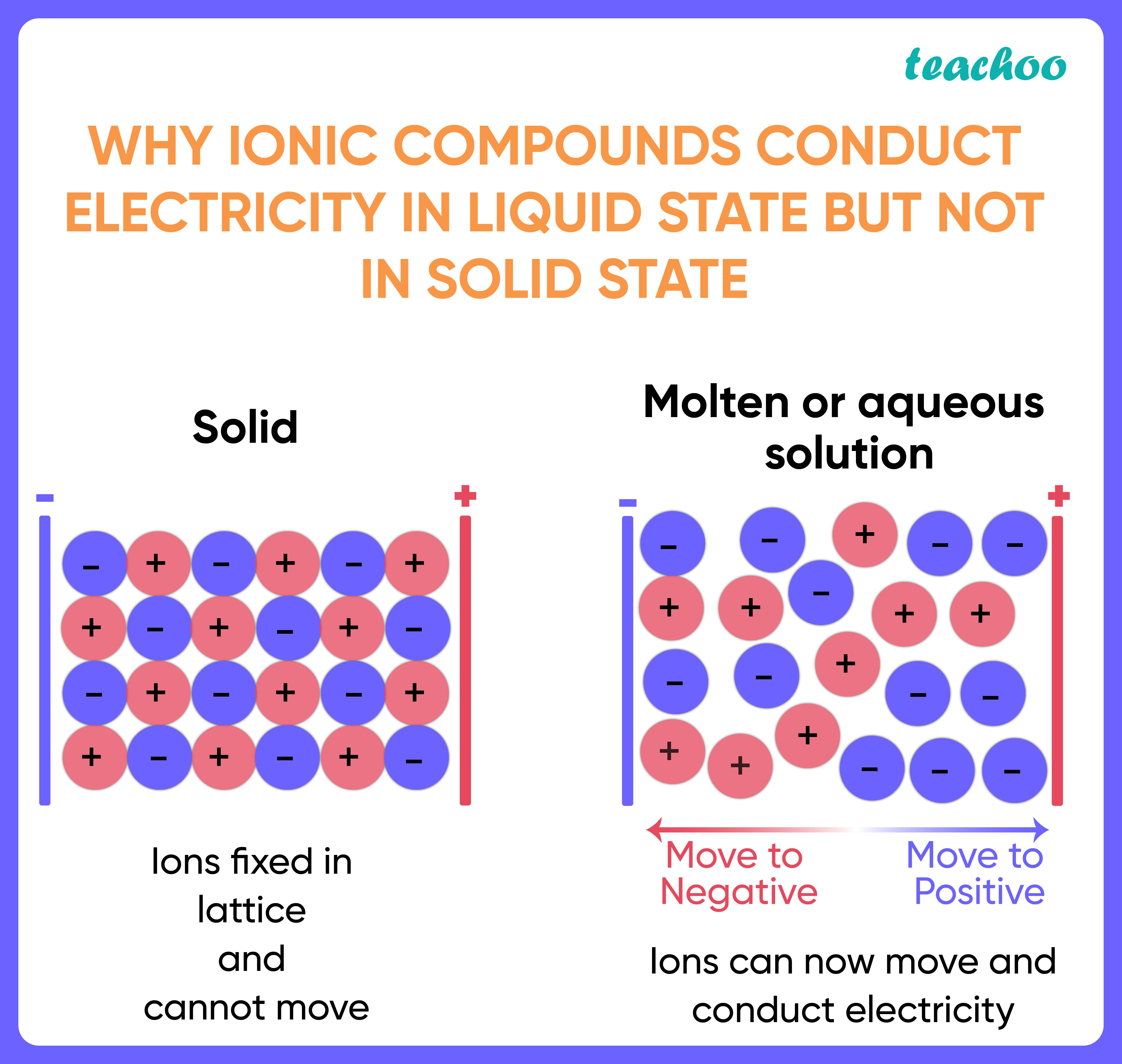
Conductors and insulators play crucial roles in various aspects of our lives, shaping the way we interact with electricity. Conductors, materials that allow electricity to flow easily through them, form the backbone of electrical systems, while insulators, materials that resist the flow of electricity, ensure safety and control.
Conductors in Electrical Systems
Conductors are essential for the transmission and distribution of electrical energy. They enable the flow of electrons, facilitating the operation of electrical devices, circuits, and networks.
- Electrical Wiring: Copper and aluminum are widely used as conductors in electrical wiring due to their excellent conductivity and affordability. These materials are used to create wires that carry electrical current to homes, businesses, and industries.
- Circuits: In electrical circuits, conductors connect various components, such as resistors, capacitors, and transistors, allowing electrical current to flow through the circuit and perform specific functions.
- Electronic Devices: Conductors are crucial in electronic devices, enabling the flow of electricity within integrated circuits, transistors, and other components. These devices rely on conductors to carry electrical signals and perform complex operations.
Insulators in Electrical Systems
Insulators are essential for safety and control in electrical systems. They prevent the flow of electricity where it is not desired, ensuring that electrical energy is directed along designated paths and preventing accidental shocks or short circuits.
- Wire Insulation: Wires are typically covered with insulating materials, such as rubber, plastic, or Teflon, to prevent accidental contact with live conductors and to prevent short circuits. This insulation ensures the safe handling and operation of electrical wiring.
- Protective Coatings: Insulating coatings are applied to electrical components and equipment to protect them from moisture, dust, and other environmental factors that could cause electrical failure. These coatings enhance the reliability and longevity of electrical systems.
Real-World Applications of Conductors and Insulators, Was leitet strom und was nicht
Conductors and insulators are ubiquitous in our daily lives, shaping the functionality of countless devices and systems.
- Household Appliances: From toasters and refrigerators to washing machines and dryers, conductors and insulators are essential for the operation of household appliances. Conductors enable the flow of electricity to power these devices, while insulators protect users from electrical hazards.
- Electronics: Conductors and insulators are integral to the functioning of smartphones, computers, televisions, and other electronic devices. Conductors carry electrical signals, while insulators prevent unwanted electrical flow and protect delicate components.
- Transportation: Conductors and insulators play crucial roles in electric vehicles, trains, and other forms of transportation. Conductors enable the flow of electricity to power motors and other systems, while insulators protect sensitive components and ensure safe operation.
Applications of Conductors and Insulators in Industries
The use of conductors and insulators varies across industries, tailored to specific needs and applications.
- Power Generation: Conductors are essential for the generation and transmission of electricity in power plants. High-voltage conductors are used to transport electrical energy from power plants to distribution networks.
- Telecommunications: Conductors are used in telecommunications networks to transmit data signals over long distances. Insulators are used to support and protect these conductors, ensuring reliable signal transmission.
- Manufacturing: Conductors and insulators are used extensively in manufacturing processes. Conductors are used in welding equipment, heating elements, and other industrial applications, while insulators provide safety and control in these processes.
Visual Representation of Conductors and Insulators in a Simple Electrical Circuit
Imagine a simple circuit with a battery, a light bulb, and connecting wires. The battery provides the electrical energy, the light bulb converts electrical energy into light, and the wires act as conductors, allowing the flow of electricity between the battery and the light bulb. The wires are covered with an insulating material, such as rubber or plastic, to prevent accidental contact with the live conductor and to prevent short circuits.
This insulation ensures the safe operation of the circuit.
The world of electricity is a captivating one, and understanding the difference between conductors and insulators is key to unlocking its mysteries. By delving into the atomic structure of materials, we can unravel the secrets behind their conductivity and learn how they shape our technological landscape. From the simplest light bulb to the most complex computer circuits, conductors and insulators work together to create the electrical wonders that define our modern world.
Clarifying Questions
What is the difference between a conductor and an insulator?
Conductors allow electricity to flow easily through them, while insulators resist the flow of electricity. This difference is determined by the availability of free electrons in the material.
Can any material be a conductor or an insulator?
While most materials are categorized as either conductors or insulators, some materials can act as both under certain conditions. For example, silicon, a semiconductor, can act as a conductor when impurities are added.
Why is copper a good conductor?
Copper has a loose outer electron that can easily move from atom to atom, making it a good conductor of electricity.
Why is rubber a good insulator?
Rubber has tightly bound electrons that are difficult to dislodge, making it a good insulator and preventing the flow of electricity.
/examples-of-electrical-conductors-and-insulators-608315_v3-5b609152c9e77c004f6e8892.png?w=1024&resize=1024,1024&ssl=1)





