How to target the cancer tumpor stroma – How to target the cancer tumor stroma? This question has become a focal point in the quest to conquer cancer. The tumor microenvironment, a complex ecosystem teeming with cells and molecules, plays a pivotal role in cancer growth and spread. While the cancer cells themselves are often the primary target of treatments, a growing body of evidence suggests that targeting the stroma, the supporting framework of the tumor, could offer a new and powerful approach to cancer therapy.
The stroma is not just a passive bystander; it actively participates in the tumor’s growth and survival. Fibroblasts, immune cells, and blood vessels within the stroma contribute to tumor angiogenesis, immune evasion, and metastasis. Understanding the intricate interplay between cancer cells and the stroma is crucial for developing effective therapies that disrupt this complex partnership.
Understanding the Tumor Microenvironment

The tumor microenvironment (TME) is a complex and dynamic ecosystem that surrounds and influences the growth and spread of cancer cells. It comprises various cell types, including cancer cells, stromal cells, and the extracellular matrix (ECM). The TME plays a critical role in tumor initiation, progression, and response to therapy.
Composition of the Tumor Microenvironment, How to target the cancer tumpor stroma
The TME is a heterogeneous mixture of cells and molecules that interact with each other in a complex and dynamic manner.
- Cancer Cells: These are the primary focus of cancer treatment. They are genetically unstable and have acquired the ability to proliferate uncontrollably, invade surrounding tissues, and metastasize to distant sites. Cancer cells are often characterized by mutations that drive their growth and survival. They also release signaling molecules that influence the behavior of other cells in the TME.
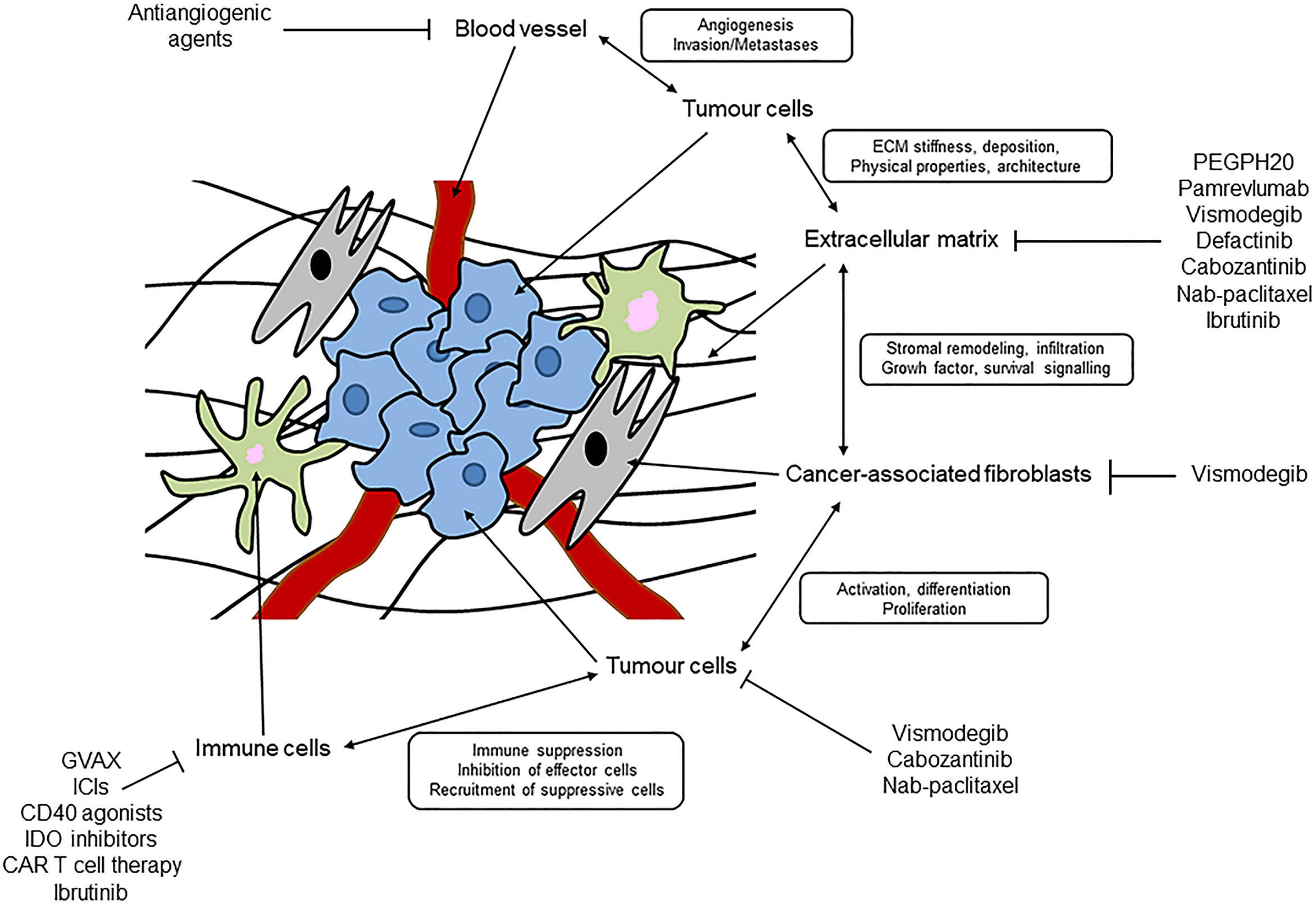
- Stromal Cells: These are non-cancerous cells that support the growth and survival of cancer cells. Stromal cells include fibroblasts, immune cells, endothelial cells, and pericytes.
- Extracellular Matrix: The ECM is a complex network of proteins and polysaccharides that provides structural support to tissues and influences cell behavior. The ECM in the TME is often remodeled by cancer cells, leading to changes in tissue architecture and promoting tumor growth and invasion.
Roles of Stromal Cells in Tumor Growth and Progression
Stromal cells play crucial roles in supporting tumor growth and progression.
- Fibroblasts: These are the most abundant stromal cell type in the TME. They produce ECM components and secrete growth factors that promote cancer cell proliferation, angiogenesis (formation of new blood vessels), and invasion. Cancer-associated fibroblasts (CAFs) are often found in the TME and are known to be highly pro-tumorigenic.
- Immune Cells: The immune system plays a complex role in cancer. Some immune cells, such as cytotoxic T lymphocytes (CTLs), can kill cancer cells. However, the TME can suppress the activity of anti-tumor immune cells, allowing cancer cells to escape immune surveillance.
- Endothelial Cells: These cells line blood vessels and are responsible for delivering nutrients and oxygen to tissues. Cancer cells can induce angiogenesis, which is the formation of new blood vessels that supply the tumor with nutrients and oxygen, allowing it to grow and spread.
Interplay Between Cancer Cells and Stromal Cells
Cancer cells and stromal cells engage in a complex interplay, influencing each other’s behavior.
- Cancer Cells Influence Stromal Cells: Cancer cells release signaling molecules, such as cytokines, chemokines, and growth factors, that can alter the behavior of stromal cells. For example, cancer cells can induce fibroblasts to produce ECM components that promote tumor growth and invasion. They can also recruit immune cells to the TME, but these immune cells can be suppressed by the TME, allowing cancer cells to evade immune destruction.
- Stromal Cells Influence Cancer Cells: Stromal cells can also influence the behavior of cancer cells. For example, fibroblasts can provide growth factors that promote cancer cell proliferation. Immune cells can kill cancer cells, but the TME can suppress the activity of anti-tumor immune cells, allowing cancer cells to escape immune surveillance.
Targeting Stromal Cells

The tumor microenvironment (TME) is a complex ecosystem that profoundly influences cancer growth and progression. While targeting cancer cells directly has been a cornerstone of cancer therapy, recent research highlights the critical role of stromal cells in supporting tumor growth and metastasis. Targeting stromal cells, therefore, emerges as a promising therapeutic strategy, aiming to disrupt the tumor’s supportive network and create a less hospitable environment for cancer cell survival.
Rationale for Targeting Stromal Cells
Stromal cells, comprising various cell types like fibroblasts, immune cells, endothelial cells, and pericytes, create the physical and biochemical framework surrounding the tumor. These cells actively participate in tumorigenesis by providing essential support for cancer cell proliferation, angiogenesis, invasion, and metastasis.
Targeting the Tumor Stroma
Targeting the tumor stroma represents a promising strategy in cancer therapy, aiming to disrupt the tumor’s supportive environment and enhance the efficacy of conventional treatments. This approach focuses on modifying the stromal components, including fibroblasts, immune cells, and the extracellular matrix, to create a less hospitable environment for tumor growth and spread.
Therapeutic Approaches to Targeting the Tumor Stroma
Several approaches are being investigated to target the tumor stroma. These strategies aim to manipulate the stromal components, either by directly targeting stromal cells or by influencing their interactions with the tumor cells.
| Approach | Mechanism | Examples | Advantages | Disadvantages |
|---|---|---|---|---|
| Stromal Cell Targeting | Directly targeting stromal cells with specific inhibitors or antibodies |
|
|
|
| Extracellular Matrix Modulation | Altering the composition and structure of the ECM to inhibit tumor growth and invasion |
|
|
|
| Immune Modulation | Manipulating the immune response within the tumor microenvironment to enhance anti-tumor immunity |
|
|
|
Ongoing Clinical Trials Investigating Stromal-Targeting Therapies
Several clinical trials are currently investigating the efficacy and safety of stromal-targeting therapies in various cancer types. These trials are evaluating a wide range of approaches, including:
- FAP inhibitors: These drugs are being investigated in combination with chemotherapy and immunotherapy in patients with solid tumors, such as pancreatic cancer and breast cancer.
- MMP inhibitors: These drugs are being evaluated in patients with advanced cancers, such as lung cancer and melanoma, to inhibit tumor invasion and metastasis.
- Checkpoint inhibitors: These drugs are being investigated in combination with other therapies, such as radiation therapy and chemotherapy, in patients with various cancers, including lung cancer, melanoma, and bladder cancer.
The results of these clinical trials will provide valuable insights into the efficacy and safety of stromal-targeting therapies and their potential to improve cancer treatment outcomes.
Targeting the Stroma for Personalized Therapy
The concept of personalized medicine, also known as precision medicine, has revolutionized the way we approach cancer treatment. This approach aims to tailor therapies to the unique characteristics of each patient and their tumor. By understanding the specific genetic and molecular features of the tumor microenvironment, including the tumor stroma, we can develop more effective and targeted therapies.
Genetic and Molecular Profiling of the Tumor Stroma
Genetic and molecular profiling of the tumor stroma provides valuable insights into the tumor’s behavior and response to therapy. This information can guide treatment decisions by identifying specific targets for therapy. For instance, analyzing the expression of genes involved in stromal cell function can reveal potential therapeutic targets.
For example, high expression of the gene COL1A1, which encodes collagen type I, a major component of the extracellular matrix, has been linked to poor prognosis in breast cancer. This finding suggests that targeting collagen production could be a viable therapeutic strategy.
Biomarkers for Predicting Response to Stromal-Targeting Therapies
Biomarkers are measurable indicators that can be used to predict the likelihood of response to a particular therapy. In the context of stromal-targeting therapies, biomarkers can help identify patients who are most likely to benefit from these treatments. These biomarkers can be genetic, molecular, or even imaging-based.
For instance, the presence of specific immune cell subtypes within the tumor stroma, such as tumor-associated macrophages (TAMs), can be used as a biomarker to predict response to immunotherapy. High levels of TAMs are often associated with poor response to immunotherapy, while low levels are associated with better outcomes.
Future Directions in Targeting the Tumor Stroma: How To Target The Cancer Tumpor Stroma
The field of stromal-targeting therapies is rapidly evolving, with exciting advancements on the horizon. Researchers are actively exploring novel approaches and technologies to enhance the efficacy and minimize the side effects of these therapies. This ongoing research holds immense promise for improving cancer treatment outcomes in the future.
Emerging Technologies and Research Areas
Several emerging technologies and research areas are poised to significantly impact the development of stromal-targeting therapies.
- Single-cell sequencing provides a detailed understanding of the heterogeneity within the tumor microenvironment, enabling the identification of specific stromal cell populations that contribute to tumor growth and metastasis. This information can be leveraged to develop targeted therapies that specifically target these cells.
- CRISPR-Cas9 gene editing allows for the precise modification of genes in stromal cells, potentially reprogramming them to suppress tumor growth or enhance anti-tumor immunity. This technology offers the possibility of developing personalized therapies tailored to the specific genetic makeup of each patient’s tumor microenvironment.
- Biomaterials and nanotechnology are being explored to develop targeted drug delivery systems that specifically deliver therapeutic agents to the tumor stroma, maximizing efficacy and minimizing off-target effects. These approaches can improve the bioavailability of drugs and enhance their penetration into the tumor mass.
- Immunotherapy holds great promise for harnessing the body’s own immune system to fight cancer. The development of immune checkpoint inhibitors and CAR T-cell therapy has revolutionized cancer treatment, and ongoing research aims to enhance the efficacy of these therapies by targeting the tumor stroma. For example, researchers are exploring ways to re-educate stromal cells to promote anti-tumor immune responses.
Combination Therapies
Combination therapies targeting both cancer cells and the tumor stroma hold significant potential for improving cancer treatment outcomes. By simultaneously targeting multiple components of the tumor microenvironment, these therapies aim to overcome resistance mechanisms and achieve synergistic effects.
- Combining stromal-targeting agents with conventional chemotherapy or radiation therapy can enhance the delivery and efficacy of these therapies by disrupting the tumor microenvironment. This approach can improve drug penetration, reduce tumor hypoxia, and enhance the sensitivity of cancer cells to treatment.
- Combining stromal-targeting agents with immunotherapy can augment the anti-tumor immune response by modulating the stromal microenvironment to favor immune cell infiltration and activation. For example, targeting stromal cells that suppress immune responses can enhance the effectiveness of checkpoint inhibitors and CAR T-cell therapy.
Novel Stromal-Targeting Agents
Researchers are actively developing novel stromal-targeting agents with enhanced efficacy and reduced side effects.
- Small molecule inhibitors targeting specific signaling pathways involved in stromal cell activation and tumor growth are being developed. These agents aim to disrupt the communication between cancer cells and stromal cells, inhibiting tumor progression.
- Antibodies targeting specific surface receptors on stromal cells are being investigated to modulate their function and suppress tumor growth. These antibodies can block the interaction of stromal cells with cancer cells, preventing tumor angiogenesis and metastasis.
- Genetically engineered stromal cells are being explored as a novel therapeutic approach. These cells can be modified to produce anti-tumor factors or deliver therapeutic agents directly to the tumor microenvironment. This approach offers the potential for personalized therapies tailored to the specific needs of each patient.
Targeting the tumor stroma represents a paradigm shift in cancer therapy, moving beyond solely focusing on cancer cells. By understanding the intricate interactions within the tumor microenvironment, researchers and clinicians are developing innovative therapies that aim to disrupt the support network that fuels tumor growth. As our knowledge of the stroma deepens, we can expect to see a wave of new therapies that target this critical component of the tumor, leading to more effective and personalized cancer treatments.
FAQ Guide
What are the challenges associated with targeting the tumor stroma?
Targeting the tumor stroma presents unique challenges. One major hurdle is the complexity of the tumor microenvironment, which varies significantly between different tumors and even within the same tumor. Another challenge is the potential for off-target effects, as therapies targeting the stroma may also affect normal tissues.
What are some examples of stromal-targeting therapies currently in clinical trials?
Several promising stromal-targeting therapies are currently being investigated in clinical trials. These include agents that inhibit angiogenesis, block immune checkpoint pathways, or target specific stromal cell populations.
How can personalized medicine be used to target the tumor stroma?
Personalized medicine approaches can be used to tailor stromal-targeting therapies to individual patients. By analyzing the genetic and molecular profile of the tumor stroma, clinicians can identify specific targets and select therapies that are most likely to be effective for that patient.






