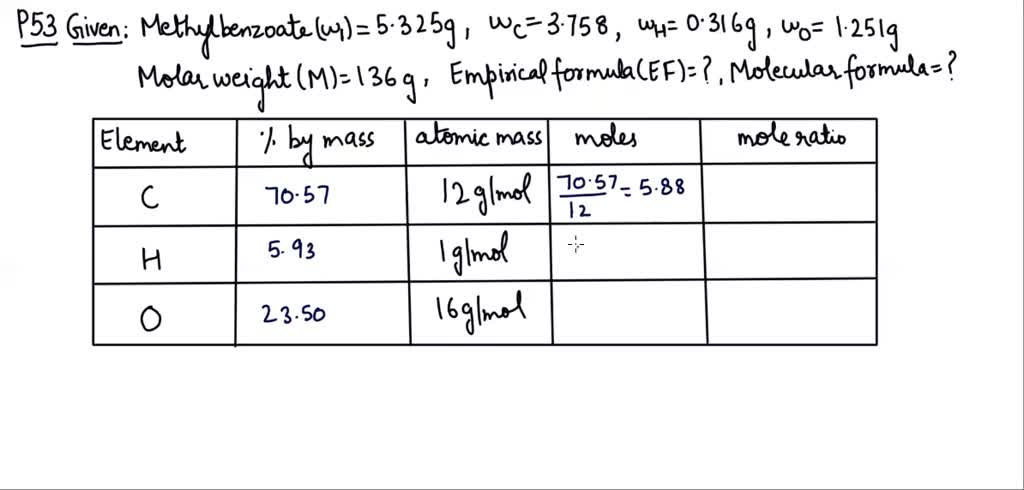
A scientist wishes to measure the concentration of methyl benzoate, a fragrant compound found in various applications. This pursuit is crucial for understanding its role in diverse fields like environmental monitoring, food safety, and industrial processes. Measuring methyl benzoate concentration accurately presents unique challenges, demanding precise analytical techniques and meticulous sample preparation.
Methyl benzoate, an aromatic ester with a sweet, floral scent, is a common ingredient in perfumes, cosmetics, and food flavorings. It also plays a significant role in industrial processes, such as the production of plastics and pharmaceuticals. Accurately measuring its concentration is vital for ensuring product quality, monitoring environmental contamination, and understanding its impact on human health.
Introduction

Methyl benzoate is an aromatic ester with the chemical formula C 8H 8O 2. It is a colorless liquid with a sweet, floral odor, reminiscent of cherry or almond. Methyl benzoate is naturally found in various fruits, flowers, and essential oils, contributing to their characteristic fragrances. Its unique properties make it valuable in various applications, including perfumery, flavoring, and pharmaceuticals.The concentration of methyl benzoate is crucial in various contexts.
In the perfume industry, its concentration determines the intensity and longevity of the fragrance. In food and beverage production, precise control of methyl benzoate concentration is essential for maintaining desired flavor profiles. In pharmaceutical applications, its concentration is critical for the effectiveness and safety of medicinal products. Accurately measuring methyl benzoate concentration presents challenges due to its volatility, potential interference from other compounds, and the need for sensitive analytical techniques.
These challenges require careful sample preparation, appropriate analytical methods, and accurate calibration procedures.
Challenges in Measuring Methyl Benzoate Concentration
The accurate measurement of methyl benzoate concentration faces several challenges:
- Volatility: Methyl benzoate is a volatile compound, meaning it readily evaporates at room temperature. This volatility can lead to sample loss during handling and analysis, affecting the accuracy of measurements.
- Interference from Other Compounds: Samples often contain other compounds that can interfere with the analysis of methyl benzoate. These interfering compounds can mask the presence of methyl benzoate or produce false-positive results.
- Sensitivity of Analytical Techniques: Accurate measurement of methyl benzoate concentration requires sensitive analytical techniques that can detect low levels of the compound. Some methods, such as gas chromatography-mass spectrometry (GC-MS), are particularly well-suited for this purpose.
Analytical Techniques: A Scientist Wishes To Measure The Concentration Of Methyl Benzoate
Measuring the concentration of methyl benzoate requires reliable and accurate analytical techniques. Various methods are employed, each with its own advantages and disadvantages, making the selection dependent on the specific application and desired level of precision.
Analytical Techniques for Methyl Benzoate Concentration Measurement
The following table summarizes common analytical techniques used for measuring methyl benzoate concentration:
| Technique | Principle | Advantages | Disadvantages |
|---|---|---|---|
| Gas Chromatography (GC) | Separation of volatile compounds based on their boiling points and interactions with a stationary phase. Detection using a flame ionization detector (FID) or mass spectrometry (MS). | High sensitivity, good resolution, quantitative analysis, wide applicability. | Requires sample preparation (e.g., extraction), may not be suitable for non-volatile compounds. |
| High-Performance Liquid Chromatography (HPLC) | Separation of compounds based on their interactions with a stationary phase and a mobile phase. Detection using a UV-Vis detector or mass spectrometry (MS). | Suitable for both volatile and non-volatile compounds, high sensitivity, good resolution, quantitative analysis. | Requires sample preparation (e.g., extraction), may be more complex than GC. |
| Spectrophotometry | Measurement of the absorbance or transmittance of light through a sample at specific wavelengths. | Simple, relatively inexpensive, rapid analysis. | Limited sensitivity, may not be suitable for complex mixtures. |
| Titration | Quantitative chemical analysis where a solution of known concentration (titrant) is added to a solution of unknown concentration (analyte) until the reaction is complete. | Simple, inexpensive, accurate for specific reactions. | Requires a specific reaction, may not be suitable for all compounds. |
GC and HPLC are particularly well-suited for analyzing complex mixtures, providing detailed information about the composition of the sample. Spectrophotometry is often used for rapid analysis of single compounds, while titration is employed for accurate quantification of specific reactions.
Sample Preparation
Proper sample preparation is crucial for accurate methyl benzoate concentration measurement. It ensures that the sample is in a suitable form for analysis and minimizes potential errors that can arise from sample heterogeneity, matrix effects, or analyte degradation.
Extraction
Extraction is a common sample preparation technique used to isolate methyl benzoate from a complex matrix. This process involves using a solvent to selectively dissolve the analyte from the sample. The choice of solvent depends on the nature of the sample and the properties of methyl benzoate. For example, a non-polar solvent like hexane or diethyl ether can be used to extract methyl benzoate from a water sample.
The extraction process can be performed using various techniques, including liquid-liquid extraction, solid-phase extraction, or microextraction.
Filtration
Filtration is a technique used to remove particulate matter from a sample. This is essential to prevent clogging of the analytical instrument or interference with the analysis. Filtration can be performed using various filters, including membrane filters, syringe filters, or filter paper. The choice of filter depends on the size of the particles to be removed and the volume of the sample.
For example, a 0.45 μm pore size filter can be used to remove particles larger than 0.45 μm from a water sample.
Dilution
Dilution is a technique used to reduce the concentration of the analyte in the sample. This is often necessary to bring the analyte concentration within the range of the analytical instrument. Dilution involves adding a known volume of solvent to a known volume of the sample.
For example, a 1:10 dilution of a water sample containing methyl benzoate would involve adding 1 mL of the sample to 9 mL of water.
Specific Sample Preparation Protocols
Sample preparation protocols vary depending on the sample matrix. Here are some examples of specific sample preparation protocols for different sample matrices:
Water Samples
For water samples, a simple filtration and dilution step may be sufficient.
- Filter the water sample using a 0.45 μm pore size filter to remove any particulate matter.
- Dilute the filtered water sample with deionized water to bring the methyl benzoate concentration within the range of the analytical instrument.
Soil Samples
Soil samples require more extensive sample preparation, including extraction and filtration.
- Extract methyl benzoate from the soil sample using a suitable solvent, such as dichloromethane or methanol.
- Filter the extract using a 0.45 μm pore size filter to remove any particulate matter.
- Dilute the filtered extract with the extraction solvent to bring the methyl benzoate concentration within the range of the analytical instrument.
Air Samples
Air samples typically involve collecting the sample on a sorbent material, followed by extraction and analysis.
- Collect the air sample on a sorbent material, such as activated carbon or Tenax TA.
- Extract methyl benzoate from the sorbent material using a suitable solvent, such as dichloromethane or methanol.
- Filter the extract using a 0.45 μm pore size filter to remove any particulate matter.
- Dilute the filtered extract with the extraction solvent to bring the methyl benzoate concentration within the range of the analytical instrument.
Calibration and Validation
Calibration is a crucial step in analytical chemistry, ensuring that the instrument used to measure the concentration of methyl benzoate provides accurate and reliable results. This process involves establishing a relationship between the instrument’s response and the known concentration of the analyte, methyl benzoate in this case.
Calibration Methods
Calibration methods are techniques used to establish this relationship between the instrument’s response and the analyte’s concentration. Different methods are employed depending on the nature of the analyte and the analytical technique used. Here are some commonly used calibration methods:
- External Calibration: This is the most straightforward method. A series of solutions with known concentrations of the analyte are prepared and measured by the instrument. The instrument’s response is then plotted against the corresponding concentrations, creating a calibration curve. The concentration of an unknown sample is then determined by measuring its response and interpolating it on the calibration curve. This method is simple and widely used, but it relies on the assumption that the instrument’s response is linear and reproducible.
- Standard Addition: This method is particularly useful when the matrix of the sample (the other components present) can interfere with the measurement. Known amounts of the analyte are added to a series of aliquots of the sample. The instrument’s response is measured for each aliquot, and the data is plotted. The concentration of the analyte in the original sample is determined by extrapolating the plot to the point where the added analyte concentration is zero.
This method helps to compensate for matrix effects, improving the accuracy of the measurement.
- Internal Standard Method: This method involves adding a known amount of a compound, known as the internal standard, to both the standards and the unknown samples. The internal standard should have a similar chemical structure and response characteristics to the analyte but should not be present in the original sample. The ratio of the analyte’s signal to the internal standard’s signal is then measured.
This ratio is less affected by variations in sample volume, injection volume, and instrument response, making it more reliable than using only the analyte’s signal. This method is particularly useful for complex matrices and when precise measurements are required.
Validation
Validation is the process of confirming that the analytical method used to measure the concentration of methyl benzoate is reliable and accurate. This involves evaluating the method’s performance characteristics, such as linearity, accuracy, precision, sensitivity, and specificity.
- Linearity: This refers to the method’s ability to produce a linear relationship between the instrument’s response and the analyte’s concentration within a specific range. Linearity is typically assessed by analyzing a series of standards with known concentrations and plotting the data. The linearity is evaluated by examining the correlation coefficient (R 2) of the calibration curve. A high R 2 value indicates a good linear relationship.
- Accuracy: Accuracy refers to the closeness of the measured value to the true value. Accuracy is typically assessed by analyzing a sample with a known concentration and comparing the measured value to the true value. The accuracy is expressed as the percentage error or the difference between the measured value and the true value.
- Precision: Precision refers to the reproducibility of the measurements. Precision is typically assessed by analyzing the same sample multiple times and measuring the standard deviation of the results. Precision is expressed as the relative standard deviation (RSD) or the coefficient of variation (CV). A lower RSD or CV indicates higher precision.
- Sensitivity: Sensitivity refers to the method’s ability to detect small amounts of the analyte. Sensitivity is typically assessed by measuring the lowest concentration of the analyte that can be reliably detected by the method. Sensitivity is expressed as the limit of detection (LOD) or the limit of quantification (LOQ).
- Specificity: Specificity refers to the method’s ability to measure only the analyte of interest and not other compounds present in the sample. Specificity is typically assessed by analyzing a sample spiked with known amounts of potential interfering compounds and observing whether the method can accurately measure the analyte without interference from the other compounds.
Data Analysis and Interpretation
After collecting the data from the analysis of methyl benzoate, the next step is to analyze and interpret the results to obtain meaningful insights. Data analysis involves using various techniques to extract information from the raw data, assess the method’s performance, and draw conclusions about the concentration of methyl benzoate in the sample.
Data Analysis Techniques
Data analysis techniques used to interpret the measured methyl benzoate concentration depend on the chosen analytical method. Common techniques include:
- Calibration Curve: A calibration curve is a graph that plots the response of the analytical instrument (e.g., absorbance, peak area) against the known concentrations of standards. The curve is used to determine the unknown concentration of the analyte in the sample by interpolating the response of the sample on the curve.
- Linear Regression: This statistical technique is used to establish a linear relationship between the response signal and the concentration of the analyte. The slope of the regression line represents the sensitivity of the method, and the intercept represents the background signal.
- Standard Addition Method: This method involves adding known amounts of the analyte to the sample and measuring the response. The increase in response is proportional to the added analyte, allowing for the determination of the unknown concentration in the original sample.
- Internal Standard Method: An internal standard is a known compound added to both the standards and the sample. By comparing the response of the analyte to the response of the internal standard, the concentration of the analyte can be determined, even if the sample volume or injection volume varies.
Assessing Method Performance
Assessing the accuracy, precision, and sensitivity of the analytical method is crucial for ensuring the reliability of the results.
- Accuracy: Accuracy refers to how close the measured value is to the true value. It is assessed by comparing the measured concentration to a known standard or reference value.
- Precision: Precision refers to the reproducibility of the measurements. It is determined by analyzing the same sample multiple times and measuring the variation in the results.
- Sensitivity: Sensitivity refers to the ability of the method to detect small changes in the concentration of the analyte. It is determined by the slope of the calibration curve, with a steeper slope indicating higher sensitivity.
Data Presentation and Interpretation
Data obtained from the analysis of methyl benzoate is typically presented in tables, graphs, and statistical summaries.
- Tables: Tables are used to present numerical data in an organized format. They can include information about the sample, the standards, the measured responses, and the calculated concentrations.
- Graphs: Graphs are used to visualize the data and identify trends. Common graphs used in analytical chemistry include calibration curves, chromatograms, and standard addition plots.
- Statistical Analysis: Statistical analysis can be used to assess the significance of the results and to determine the uncertainty associated with the measurements. Common statistical tests include t-tests, ANOVA, and regression analysis.
For example, a calibration curve can be used to determine the concentration of methyl benzoate in a sample by plotting the absorbance of known standards against their concentrations. The absorbance of the sample can then be interpolated on the curve to obtain the corresponding concentration.
Applications
Methyl benzoate concentration measurement finds wide-ranging applications across diverse fields, playing a crucial role in ensuring safety, quality, and environmental protection. The following sections explore specific applications in environmental monitoring, food safety, pharmaceutical analysis, and industrial process control.
Environmental Monitoring, A scientist wishes to measure the concentration of methyl benzoate
Monitoring methyl benzoate levels in the environment is essential for assessing potential risks to human health and ecosystems. Methyl benzoate, a common fragrance ingredient, can be released into the environment through various sources, including industrial emissions, wastewater discharges, and the degradation of pesticides.
- Air Quality Monitoring: Methyl benzoate can be present in ambient air as a volatile organic compound (VOC). Monitoring its concentration in air helps assess potential air pollution and health risks associated with its inhalation.
- Water Quality Monitoring: Methyl benzoate can be released into water bodies through industrial discharges or agricultural runoff. Monitoring its concentration in water helps assess potential contamination of drinking water sources and aquatic ecosystems.
- Soil Contamination Assessment: Methyl benzoate can persist in soil, potentially impacting soil health and plant growth. Monitoring its concentration in soil helps assess the extent of contamination and guide remediation efforts.
Food Safety
Methyl benzoate is a natural component of some foods and is also used as a flavoring agent. Monitoring its concentration in food is essential for ensuring food safety and quality.
- Food Processing: Methyl benzoate is used as a flavoring agent in various food products, including beverages, candies, and baked goods. Monitoring its concentration during food processing helps ensure that it is added at safe levels and does not exceed regulatory limits.
- Food Packaging: Methyl benzoate can migrate from food packaging materials into food products. Monitoring its concentration in food packaging helps assess potential contamination of food and ensure that packaging materials are safe for food contact.
Pharmaceutical Analysis
Methyl benzoate is a common ingredient in pharmaceuticals, used as a solvent, preservative, or flavoring agent. Monitoring its concentration in pharmaceutical products is essential for ensuring product quality and safety.
- Drug Formulation: Methyl benzoate is used as a solvent in the formulation of some drugs. Monitoring its concentration in drug formulations helps ensure that it is present at the correct levels for optimal drug delivery and efficacy.
- Drug Stability: Methyl benzoate can degrade over time, potentially affecting the stability and efficacy of pharmaceutical products. Monitoring its concentration in drug products helps assess their stability and ensure that they remain safe and effective for their intended use.
Industrial Process Control
Methyl benzoate is used as an intermediate in the production of various chemicals, including pharmaceuticals, pesticides, and fragrances. Monitoring its concentration in industrial processes is essential for optimizing production efficiency and ensuring product quality.
- Reaction Monitoring: Methyl benzoate is often produced through chemical reactions. Monitoring its concentration during these reactions helps ensure that the reaction proceeds efficiently and that the desired product is formed in the correct quantities.
- Product Quality Control: Methyl benzoate is a key component in many industrial products. Monitoring its concentration in these products helps ensure that they meet quality standards and are safe for their intended use.
Safety Considerations
Methyl benzoate, while generally considered safe when handled appropriately, poses certain risks that require careful attention. Understanding the potential hazards associated with methyl benzoate and implementing appropriate safety precautions are crucial for ensuring the well-being of individuals working with this compound.
Potential Hazards of Methyl Benzoate Exposure
Exposure to methyl benzoate can occur through inhalation, skin contact, or ingestion. The severity of effects depends on the concentration and duration of exposure.
- Inhalation: Inhaling methyl benzoate vapors can irritate the respiratory system, leading to coughing, shortness of breath, and potentially more severe respiratory problems.
- Skin Contact: Direct contact with methyl benzoate can cause skin irritation, redness, and itching. In some cases, prolonged or repeated contact may lead to dermatitis.
- Ingestion: Ingesting methyl benzoate can result in gastrointestinal upset, including nausea, vomiting, and abdominal pain. In severe cases, it may lead to more serious health complications.
Safety Precautions and Handling Procedures
To minimize the risk of exposure and ensure safe handling of methyl benzoate, the following precautions should be implemented:
- Work in a well-ventilated area: This helps to minimize the concentration of methyl benzoate vapors in the air.
- Use appropriate personal protective equipment (PPE): Wear gloves, lab coat, and safety goggles to prevent skin and eye contact. A respirator may be necessary if working with high concentrations of methyl benzoate.
- Avoid skin and eye contact: Wash hands thoroughly after handling methyl benzoate and avoid touching your face or eyes.
- Store methyl benzoate in a cool, dry, and well-ventilated area: Keep it away from heat and direct sunlight.
- Label containers clearly: Identify the contents and any associated hazards.
- Follow proper waste disposal guidelines: Dispose of methyl benzoate and its solutions according to local regulations and safety protocols.
Personal Protective Equipment
The selection of appropriate PPE is essential for safeguarding individuals from potential hazards associated with methyl benzoate.
- Gloves: Gloves made of nitrile or neoprene are recommended for handling methyl benzoate. The choice of glove material depends on the specific application and concentration of the compound.
- Lab Coat: A long-sleeved lab coat provides a barrier against skin contact with methyl benzoate.
- Safety Goggles: Safety goggles with side shields are essential to protect the eyes from splashes or fumes.
- Respirator: A respirator with an appropriate cartridge or filter may be necessary if working with high concentrations of methyl benzoate vapors. The type of respirator should be selected based on the concentration of vapors and the duration of exposure.
Waste Disposal
Proper disposal of methyl benzoate and its solutions is crucial to prevent environmental contamination and ensure safety.
- Follow local regulations: Dispose of methyl benzoate and its solutions according to local regulations and safety protocols.
- Never pour methyl benzoate down the drain: It can harm aquatic life and contaminate water sources.
- Use appropriate containers: Dispose of methyl benzoate in designated waste containers or according to specific instructions provided by your institution or local authorities.
The journey of a scientist seeking to measure methyl benzoate concentration involves a delicate dance between analytical prowess and meticulous attention to detail. From choosing the right analytical technique to mastering sample preparation and data interpretation, each step contributes to achieving accurate and reliable results. The insights gained from these measurements are crucial for safeguarding our environment, ensuring food safety, and advancing scientific understanding in various fields.
FAQ Summary
What are the common sources of methyl benzoate contamination?
Methyl benzoate can contaminate the environment through industrial emissions, agricultural practices, and the breakdown of certain pesticides.
What are the health risks associated with methyl benzoate exposure?
High levels of methyl benzoate exposure can cause skin and eye irritation, respiratory problems, and potential liver damage.
How is methyl benzoate concentration measured in food samples?
Gas chromatography-mass spectrometry (GC-MS) is a commonly used technique for measuring methyl benzoate concentration in food samples. This method allows for the identification and quantification of various volatile compounds, including methyl benzoate.







